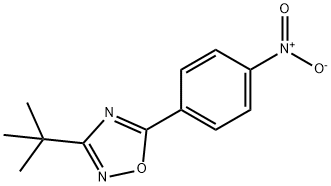
3-(tert-Butyl)-5-(4-nitrophenyl)-1,2,4-oxadiazole synthesis
- Product Name:3-(tert-Butyl)-5-(4-nitrophenyl)-1,2,4-oxadiazole
- CAS Number:1135282-84-6
- Molecular formula:C12H13N3O3
- Molecular Weight:247.25
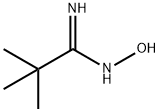
42956-75-2
37 suppliers
$60.00/100mg
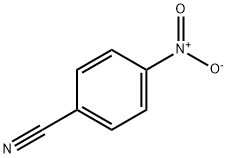
619-72-7
346 suppliers
$11.00/1g

1135282-84-6
7 suppliers
inquiry
Yield:1135282-84-6 94%
Reaction Conditions:
with mesitylene sulfonic acid;zinc dibromide in acetonitrile at 80; for 2 h;Inert atmosphere;Reagent/catalyst;Solvent;Time;
Steps:
Representative procedure for the synthesis of 3-tertbutyl-5-(4-nitrophenyl)-1,2,4-oxadiazole (2)
To a mixture of 0.116 g (1 mmol) of tert-butylamidoxime inacetonitrile under an inert atmosphere was added 0.150 g(1 mmol) of 4-nitrobenzonitrile in molar ratio 1:1. To this reactionmixture was added 0.3 mmol (0.07 g) of 2-mesitylenesulfonicacid and 0.3 mmol (0.067 g) of ZnBr2, and the mixturewas heated to 80 °C for 2 hours. After the reaction was finishedthe heating was removed, and the mixture was cooled to roomtemperature. The solvent was concentrated under reduced pressure,and ethyl acetate was added. The mixture was washedwith sodium hydrogen carbonate solution, water and brine. Theorganic phase was dried over anhydrous sodium sulfate,filtered, and the solvent was removed. The resulting residue wasdried in vacuum and flash chromatographed (silica, ethylacetate/hexane 2:1), yielding after purification 0.232 g(0.94 mmol, 94%) of 2 as a white solid. MS m/z: 247 (M+, 25),232 (45), 150 (100); 1H NMR (200 MHz, CDCl3) δ 8.43-8.28(m, 4H), 1.45 (s, 9H); 13C NMR (50 MHz, CDCl3) δ 28.4, 32.6,124.2, 129.9, 144.6, 148.9, 174.2, 178.4.
References:
Maftei, Catalin V.;Fodor, Elena;Jones, Peter G.;Franz, M. Heiko;Kelter, Gerhard;Fiebig, Heiner;Neda, Ion [Beilstein Journal of Organic Chemistry,2013,vol. 9,p. 2202 - 2215]