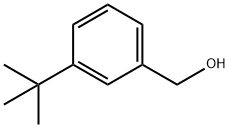
(3-tert-butylphenyl)Methanol synthesis
- Product Name:(3-tert-butylphenyl)Methanol
- CAS Number:51503-09-4
- Molecular formula:C11H16O
- Molecular Weight:164.25
27330-57-0
11 suppliers
$70.68/100mgs:

51503-09-4
16 suppliers
$279.23/1gm:
Yield:51503-09-4 100%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 20; for 2 h;Inert atmosphere;
Steps:
4.1.2.5. 3-tert-Butylbenzyl bromide (7d)
3-t-Butylphenol (1.00 g, 6.66 mmol) was dissolved in anhydrous CH2Cl2 (130 mL) and cooled to 0 °C in an ice bath. 2,6-Lutidine (1.7 mL, 14.64 mmol) and triflic anhydride (2.2 mL, 13.31 mmol) were added successively. The mixture was stirred overnight at room temperature, quenched with water and extracted with CH2Cl2. The organic phase was washed successively with aqueous saturated NaHCO3 solution and brine, dried with MgSO4, filtered and evaporated to yield 1.109 g (59%) of 7a. 1H NMR δ (CDCl3) 1.33 (s, 9H, t-butyl), 7.09 (ddd, 1H, J1 = 1.4 Hz, J2 = 2.2 Hz, J3 = 7.8 Hz 4-CH), 7.25 (t, 1H, J1 = 2.0 Hz, 2-CH), 7.40 (m, 2H, 5-CH and 6-CH). The triflic ester 7a (1.11 g, 3.93 mmol) was dissolved in anhydrous DMF (150 mL) and anhydrous MeOH (50 mL). Et3N (1.6 mL, 11.76 mmol), Pd(OAc)2 (265 mg, 1.18 mmol) and 1,3-bis(diphenylphosphino) propane (486 mg, 1.18 mmol) were added. Gaseous CO was bubbled for 1 h through the mixture while it was heated to 90 °C. The reaction mixture was then stirred overnight at room temperature under a CO atmosphere, after which it was poured into brine and extracted with Et2O. The organic phase was washed with water, brine, dried over MgSO4, filtered and evaporated. The crude product was purified by flash chromatography on silica gel using hexanes/EtOAc (95/5) to yield 554 mg (73%) of 7b. 1H NMR δ (CDCl3) 1.35 (s, 9H, t-butyl), 3.92 (s, 3H, COOCH3), 7.37 (t, 1H, J = 7.8 Hz, 5-CH), 7.60 (dq, 1H, J1 = 1.2 Hz, J2 = 7.9 Hz, 4-CH), 7.86 (dt, 1H, J1 = 1.3 Hz, J2 = 7.7 Hz, 6-CH), 8.08 (t, 1H, J = 1.8 Hz, 2-CH). The ester 7b (554 mg, 2.88 mmol) was dissolved in anhydrous THF (30 mL) and cooled to 0 °C. Lithium aluminum hydride (219 mg, 5.76 mmol) was added in small portions and the mixture stirred 2 h under argon at room temperature. Water was then added, concentrated HCl added to acidify the solution and the mixture was extracted with Et2O, dried over MgSO4, filtered and concentrated to achieve quantitatively 7c. 1H NMR δ (CDCl3) 1.34 (s, 9H, t-butyl), 4.70 (s, 2H, PhCH2OH), 7.19 (d, 1H, J1 = 6.9 Hz, 6-CH), 7.33 (m, 2H, 4-CH and 5-CH), 7.40 (s, 1H, 2-CH).
References:
Fournier, Diane;Poirier, Donald [European Journal of Medicinal Chemistry,2011,vol. 46,# 9,p. 4227 - 4237] Location in patent:experimental part
23039-28-3
75 suppliers
$16.00/250mg

51503-09-4
16 suppliers
$279.23/1gm:
![6-Oxatricyclo[3.2.1.02,7]octan-8-ol, 8-(1,1-dimethylethyl)-, (1α,2β,5α,7β,8R*)- (9CI)](/CAS/20210305/GIF/103668-93-5.gif)
103668-93-5
0 suppliers
inquiry

51503-09-4
16 suppliers
$279.23/1gm:

102405-32-3
11 suppliers
inquiry

102405-32-3
11 suppliers
inquiry

51503-09-4
16 suppliers
$279.23/1gm:
![6-Oxatricyclo[3.2.1.02,7]octan-8-one](/CAS/20200611/GIF/103668-90-2.gif)
103668-90-2
0 suppliers
inquiry

51503-09-4
16 suppliers
$279.23/1gm: