1-BENZYL-N-METHOXY-N-METHYLPIPERIDINE-4-CARBOXAMIDE synthesis
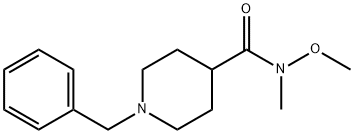
- Product Name:1-BENZYL-N-METHOXY-N-METHYLPIPERIDINE-4-CARBOXAMIDE
- CAS Number:301219-96-5
- Molecular formula:C15H22N2O2
- Molecular Weight:262.35
Yield:-
Reaction Conditions:
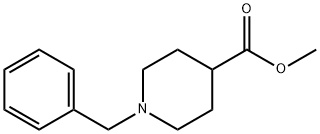
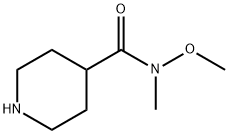
Stage #1: 1-benzylpiperidine-4-carboxylic acid methyl ester;N,O-dimethylhydroxylamine*hydrochloridewith isopropylmagnesium bromide in tetrahydrofuran at -20 - 5; for 0.666667 h;
Stage #2: with water;ammonium chloride in tetrahydrofuran;
Steps:
1.1.5
1.5 (1-Benzylpiperidin-4-yl)-N-methoxy-N-methylcarboxamide A solution of 4.17 g (42.8 mmol) of N,O-dimethylhydroxylamine hydrochloride in 195 mL of THF is cooled to -20° C., and 6.51 g (28 mmol) of methyl (1-benzylpiperidin-4-yl)carboxylate are added. Next, 85 mL of a 1M solution of isopropylmagnesium bromide in THF are added over 20 minutes while keeping the temperature in the region of 5° C. The mixture is stirred at -10° C. for 20 minutes. The reaction medium is hydrolyzed with 40 mL of 20% ammonium chloride solution and then extracted with ethyl acetate. The combined organic phases are washed with water and with brine, and then dried over anhydrous sodium sulfate and evaporated under reduced pressure. 6.9 g of an oily product are obtained.1H NMR (DMSO-d6, 250 MHz) δ ppm: 1.5-1.75 (m, 4H); 1.9-2.05 (m, 2H); 2.55-2.7 (m, 1H); 2.8-2.9 (m, 2H); 3.09 (s, 3H); 3.46 (s, 2H); 3.67 (s, 3H); 7.2-7.4 (m, 5H).
References:
US2009/124624,2009,A1 Location in patent:Page/Page column 17

6638-79-5
589 suppliers
$6.00/25g
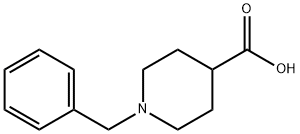
10315-07-8
133 suppliers
$10.00/250mg

301219-96-5
4 suppliers
inquiry