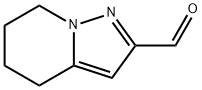
Pyrazolo[1,5-a]pyridine-2-carboxaldehyde, 4,5,6,7-tetrahydro- (9CI) synthesis
- Product Name:Pyrazolo[1,5-a]pyridine-2-carboxaldehyde, 4,5,6,7-tetrahydro- (9CI)
- CAS Number:307313-06-0
- Molecular formula:C8H10N2O
- Molecular Weight:150.18
![(4,5,6,7-TETRAHYDROPYRAZOLO[1,5-A]PYRIDIN-2-YL)METHANOL](/CAS/GIF/623564-49-8.gif)
623564-49-8
15 suppliers
inquiry
![Pyrazolo[1,5-a]pyridine-2-carboxaldehyde, 4,5,6,7-tetrahydro- (9CI)](/CAS/GIF/307313-06-0.gif)
307313-06-0
19 suppliers
$65.00/10mg
Yield:307313-06-0 77%
Reaction Conditions:
with manganese(IV) oxide in chloroform; for 1 h;Heating / reflux;
Steps:
9.4
Step 4: 4, 5. 6, 7-Tetrahvdronvrazolof1. 5-alpyridine-2-carbaldehyde; Mn02 (activated) (3.36 g) was added to the CHCI3 (44 mL) solution of (4,5, 6, 7-tetrahydropyrazolo [1,5-a] pyridin-2-yl) methanol (673 mg) and refluxed for 1 h under a nitrogen atmosphere. The reaction mixture was filtered through a pad of Celite. The filtrate was reduced under reduced pressure. The residue was applied to silica gel column chromatography, then the column was eluted with n-hexane- AcOEt (2/1-1/2). The titled compound was obtained as pale yellow oil (510 mg, 77%). 'H NMR (CDCI3) 81. 90 (m, 2H), 2.10 (m, 2H), 2.84 (t, 2H, J = 6.4 Hz), 4.23 (t, 2H, J = 6. 2 Hz), 6.52 (s, 1H), 9.92 (s, 1H).
References:
WO2003/93279,2003,A1 Location in patent:Page/Page column 70
![1H-Pyrazole-3-carboxaldehyde, 1-[4-(phenylseleno)butyl]-](/CAS/20210305/GIF/1052103-62-4.gif)
1052103-62-4
0 suppliers
inquiry
![Pyrazolo[1,5-a]pyridine-2-carboxaldehyde, 4,5,6,7-tetrahydro- (9CI)](/CAS/GIF/307313-06-0.gif)
307313-06-0
19 suppliers
$65.00/10mg
![ethyl 4,5,6,7-tetrahydropyrazolo[1,5-a]pyridine-2-carboxylate](/CAS2/GIF/307307-84-2.gif)
307307-84-2
39 suppliers
$303.00/1g
![Pyrazolo[1,5-a]pyridine-2-carboxaldehyde, 4,5,6,7-tetrahydro- (9CI)](/CAS/GIF/307313-06-0.gif)
307313-06-0
19 suppliers
$65.00/10mg
![4,5,6,7-Tetrahydro-3-hydroxy-[1,2,3]oxadiazolo[3,4-a]pyridin-8-ium inner salt](/CAS/GIF/105786-95-6.gif)
105786-95-6
39 suppliers
$185.00/100mg
![Pyrazolo[1,5-a]pyridine-2-carboxaldehyde, 4,5,6,7-tetrahydro- (9CI)](/CAS/GIF/307313-06-0.gif)
307313-06-0
19 suppliers
$65.00/10mg
4515-18-8
18 suppliers
inquiry
![Pyrazolo[1,5-a]pyridine-2-carboxaldehyde, 4,5,6,7-tetrahydro- (9CI)](/CAS/GIF/307313-06-0.gif)
307313-06-0
19 suppliers
$65.00/10mg