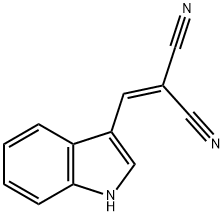
2-amino-4-(1H-indol-3-yl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile synthesis
- Product Name:2-amino-4-(1H-indol-3-yl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile
- CAS Number:307552-88-1
- Molecular formula:C20H19N3O2
- Molecular Weight:333.38
487-89-8
626 suppliers
$6.00/10g
126-81-8
424 suppliers
$5.00/10g

109-77-3
453 suppliers
$10.00/5g

307552-88-1
10 suppliers
inquiry
Yield:307552-88-1 92%
Reaction Conditions:
with palladium acetate-functionalized silica-coated Fe3O4 magnetic nanoparticles in ethanol at 20; for 0.333333 h;Green chemistry;
Steps:
2.6. General procedure for the synthesis of tetrahydro-4H-chromenes (6a-o), 2-amino-4H-pyrans (7a-o), and spirooxindole derivatives (8a-d)
General procedure: A mixture of aromatic aldehyde or isatins (1.0 mmol), mal- ononitrile (1.0 mmol), dimedone, methylaceto acetate and a cat- alytic amount of (10 mg) in ethanol was stirred at room temper- ature. The progress of the reaction was monitored by thin-layer chromatography (TLC). During the reaction, solid observed in flask within 2-5 min and after complete conversion, the reaction mass was transferred to an ice-water mixture under vigorous stirring. After stirring at room temperature, the solid was collected by fil- tration, washed with cold aq. ethanol, and dried. The authentic- ity of products was established by comparing their melting points with those reported in the literature and by the spectral data of FT-IR, 1 H and 13 C NMR and mass analysis.
References:
Poor Heravi, Mohammad Reza;Aghamohammadi, Parinaz;Vessally, Esmail [Journal of Molecular Structure,2022,vol. 1249,art. no. 131534]