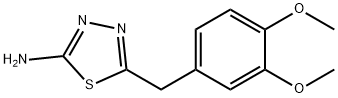
5-(3,4-DIMETHOXY-BENZYL)-[1,3,4]THIADIAZOL-2-YLAMINE synthesis
- Product Name:5-(3,4-DIMETHOXY-BENZYL)-[1,3,4]THIADIAZOL-2-YLAMINE
- CAS Number:313957-85-6
- Molecular formula:C11H13N3O2S
- Molecular Weight:251.3
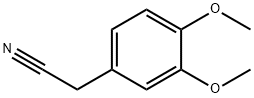
93-17-4
414 suppliers
$7.00/10g
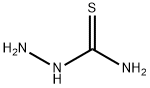
79-19-6
476 suppliers
$14.00/1g
![5-(3,4-DIMETHOXY-BENZYL)-[1,3,4]THIADIAZOL-2-YLAMINE](/CAS/GIF/313957-85-6.gif)
313957-85-6
37 suppliers
$57.50/250mg
Yield:313957-85-6 87%
Reaction Conditions:
with trifluoroacetic acid at 60;Reflux;
Steps:
2.3 General method for the synthesis of mono 2-amino-1,3,4-thiadiazole derivatives
General procedure: In a round-bottomed flask, compounds (1aec) (0.002 mol) andthiosemicarbazide (0.003 mol) in trifluoroacetic acid (5 mL) at60 C were stirred for 3e5 h. The progress of the reaction wasmonitored by TLC at appropriate time intervals. After completion ofthe reaction, the mixture was poured into 200 mL of ice-cold waterand neutralized with ammonia. The solution was filtered and thesolid matter was obtained. It was washed with deionized water,ethanol and diethyl ether, respectively. The solid was recrystallizedfrom the appropriate solvent. The physical properties and spectraldata derived from the obtained products are listed below.
References:
Er, Mustafa;Isildak, Gamze;Tahtaci, Hakan;Karakurt, Tuncay [Journal of Molecular Structure,2016,vol. 1110,p. 102 - 113]