
3-(BENZO[4,5]THIAZOLO[2,3-C][1,2,4]TRIAZOL-3-YLSULFANYL)-PROPIONIC ACID synthesis
- Product Name:3-(BENZO[4,5]THIAZOLO[2,3-C][1,2,4]TRIAZOL-3-YLSULFANYL)-PROPIONIC ACID
- CAS Number:314245-25-5
- Molecular formula:C11H9N3O2S2
- Molecular Weight:279.34
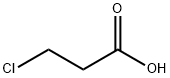
107-94-8
223 suppliers
$9.00/1g
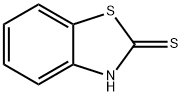
![2H-BENZO[4,5]THIAZOLO[2,3-C][1,2,4]TRIAZOLE-3-THIONE](/CAS/GIF/6957-85-3.gif)
6957-85-3
26 suppliers
$45.00/50mg
![3-(BENZO[4,5]THIAZOLO[2,3-C][1,2,4]TRIAZOL-3-YLSULFANYL)-PROPIONIC ACID](/StructureFile/ChemBookStructure4/GIF/CB4190125.gif)
314245-25-5
12 suppliers
$378.00/1g
Yield: 79.2%
Reaction Conditions:
with sodium hydroxide in ethanol;water for 12 h;Reflux;
Steps:
9 3.2.9 Synthesis of β-(s-triazolo[3,4-b]benzothiazol-3-ylthio)propionic acid (9)
To the solution of s-triazolo[3,4-b]benzothiazole-3-thiol (3) (1.0 g, 4.8 mmol) in aqueous ethanol (20 mL), sodium hydroxide (0.38 g, 4.8 mmol) and β-chloropropionic acid (0.52 g, 4.8 mmol) were added. The reaction mixture was refluxed for 12 h, cooled, filtered, and acidified with conc. HCl. The formed precipitate was filtered off, washed with cold water, and recrystallization from aqueous ethanol to yield 9 as white amorphous powder (1.10 g, 79.2% yield), Rf = 0.12 (S1), m.p. 209-211 °C. 1H NMR (300 MHz, DMSO): δ 12.40 (1H, brs, OH), 8.13-8.05 (2H, m, ArH), 7.62-7.51 (2H, m, ArH), 3.39-3.35 (2H, t, J = 6.9 Hz, SCH2), 2.77-2.72 (2H, t, J = 6.6 Hz, CH2CO). 13C NMR (75 MHz, DMSO): δ 172.9 (CO), 156.6, 143.1 (2SCN), 131.9, 129.9, 127.4, 126.7, 125.9, 114.5 (Ar-C), 34.5 (NCH2), 29.3 (CH2). IR (KBr, ν/cm-1) 3500-2600 (OH), 1735 (C=O, acid). MS (m/z): 279 [M]+. Anal. Calcd. for C11H9N3O2S2 (279.34): C, 47.30; H, 3.25; N, 15.04; found C, 47.64; H, 3.30; N, 14.97.
References:
Aboelmagd;Ali, Ibrahim A.I.;Salem, Ezzeldin M.S.;Abdel-Razik [European Journal of Medicinal Chemistry,2013,vol. 60,p. 503 - 511]
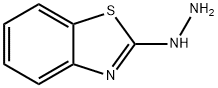
615-21-4
220 suppliers
$45.00/10mg
![3-(BENZO[4,5]THIAZOLO[2,3-C][1,2,4]TRIAZOL-3-YLSULFANYL)-PROPIONIC ACID](/StructureFile/ChemBookStructure4/GIF/CB4190125.gif)
314245-25-5
12 suppliers
$378.00/1g
149-30-4
672 suppliers
$5.00/25g
![3-(BENZO[4,5]THIAZOLO[2,3-C][1,2,4]TRIAZOL-3-YLSULFANYL)-PROPIONIC ACID](/StructureFile/ChemBookStructure4/GIF/CB4190125.gif)
314245-25-5
12 suppliers
$378.00/1g