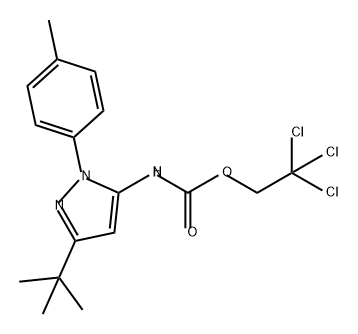
2,2,2-trichloroethyl 3-tert-butyl-1-(4-methylphenyl)-1H-pyrazol-5-ylcarbamate synthesis
- Product Name:2,2,2-trichloroethyl 3-tert-butyl-1-(4-methylphenyl)-1H-pyrazol-5-ylcarbamate
- CAS Number:317806-87-4
- Molecular formula:C17H20Cl3N3O2
- Molecular Weight:404.72
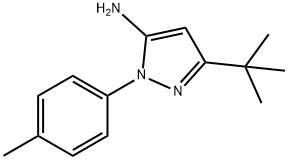
285984-25-0
108 suppliers
$5.00/100mg
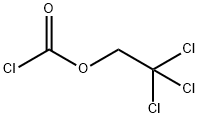
17341-93-4
310 suppliers
$11.00/1g

317806-87-4
15 suppliers
$285.00/0.1/ G
Yield:317806-87-4 99%
Reaction Conditions:
with sodium hydroxide in lithium hydroxide monohydrate;ethyl acetate;
Steps:
134.D Preparation of 2-(5-{2-[3-(5-tert-butyl-2-p-tolyl-2H-pyrazol-3-yl)-ureidomethyl]-4-fluorophenoxy}-indazol-1-yl)-N,N-dimethylacetamide (47d)
Step D: (5-tert-Butyl-2-p-tolyl-2H-pyrazol-3-yl)-carbamic acid 2,2,2-trichloroethyl ester A cooled (0° C.) biphasic solution of 5-tert-butyl-2-p-tolyl-2H-pyrazol-3-ylamine (26.6 g, 100 mmol) in water (80 mL) and ethyl acetate (180 mL) was treated with NaOH (10 g, 250 mmol) followed by trichloroethylchloroformate (29.7 g, 140 mmol). The reaction mixture was warmed to room temperature and stirred for 1 hour. The layers were separated and the organic layer was washed with brine (100 mL), dried over MgSO4, filtered through Celite, and concentrated under reduced pressure to provide 40.3 g of pale yellow solid (99% yield).
References:
US2004/192653,2004,A1
59997-51-2
270 suppliers
$6.00/25g

317806-87-4
15 suppliers
$285.00/0.1/ G
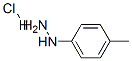
637-60-5
322 suppliers
$6.00/5g

317806-87-4
15 suppliers
$285.00/0.1/ G
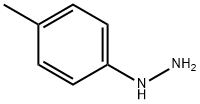
539-44-6
77 suppliers
inquiry

317806-87-4
15 suppliers
$285.00/0.1/ G