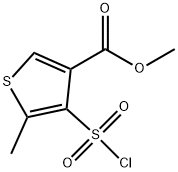
3-Thiophenecarboxylic acid, 4-(chlorosulfonyl)-5-methyl-, methyl ester synthesis
- Product Name:3-Thiophenecarboxylic acid, 4-(chlorosulfonyl)-5-methyl-, methyl ester
- CAS Number:317815-94-4
- Molecular formula:C7H7ClO4S2
- Molecular Weight:254.71
81528-48-5
48 suppliers
$16.00/250mg

317815-94-4
1 suppliers
inquiry
Yield:317815-94-4 81%
Reaction Conditions:
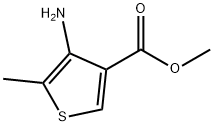
Stage #1: methyl 4-amino-5-methylthiophen-3-carboxylatewith hydrogenchloride;sodium nitrite in water at 0 - 5; for 1 h;
Stage #2: with sulfur dioxide;copper(l) iodide;dodecyltrimethylammonium bromide in dichloromethane;water at 20 - 40; for 13 h;
Stage #3: with hydrogenchloride in dichloromethane;water at 20; for 4 h;
Steps:
V-1.1
Example V-1); At from 0° C. to 5° C., a solution of 19.9 g (0.29 mol) of sodium nitrite in 60 ml of water is added dropwise with stirring to a solution of 42.7 g (0.25 mol) of methyl 3-amino-2-methyl-thiophene-4-carboxylate in 75 ml of 10% strength aqueous hydrochloric acid. The reaction mixture is stirred at from 0° C. to 5° C. for 60 minutes. The excess of nitrite is subsequently destroyed using amidosulphonic acid. At from 0° C. to 5° C., the mixture is then added dropwise with stirring to a solution of 35 g (0.55 mol) of sulphur dioxide in 300 ml of methylene chloride. After addition of 1.5 g of copper(I) chloride and 1.5 g of dodecyl-trimethylammonium bromide, the reaction mixture is stirred at 40° C. for 60 minutes and then at 20° C. for 12 hours. 18 ml of 35% strength aqueous hydrochloric acid are then added, the mixture is stirred at 20° C. for 4 hours and the phases are then separated. The aqueous phase is re-extracted with methylene chloride and the combined organic phases are washed with water, dried with magnesium sulphate and filtered. The filtrate is concentrated under water-pump vacuum and the residue is crystallized from hexane.This gives 51.7 g (81% of theory) of 4-methoxycarbonyl-2-methyl-thiophene-3-sulphonyl chloride. A mixture of 45 g (177 mmol) of 4-methoxycarbonyl-2-methyl-thiophene-3-sulphonyl chloride, 34 g (354 mmol) of ammonium carbonate and 400 ml of methylene chloride is stirred at room temperature (about 20° C.) for 12 hours. The mixture is filtered and the solvent is distilled off from the filtrate under water-pump vacuum, the residue is digested with diethyl ether and the crystalline product is isolated by filtration with suction.This gives 21.5 g (52% of theory) of 4-methoxycarbonyl-2-methyl-thiophene-3-sulphonamide
References:
US6887831,2005,B1 Location in patent:Page/Page column 21-22