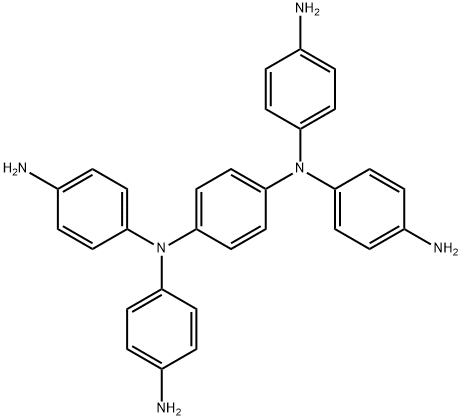
N,N,N',N'-Tetrakis(4-aminophenyl)-1,4-benzenediamine synthesis
- Product Name:N,N,N',N'-Tetrakis(4-aminophenyl)-1,4-benzenediamine
- CAS Number:3283-07-6
- Molecular formula:C30H28N6
- Molecular Weight:472.58
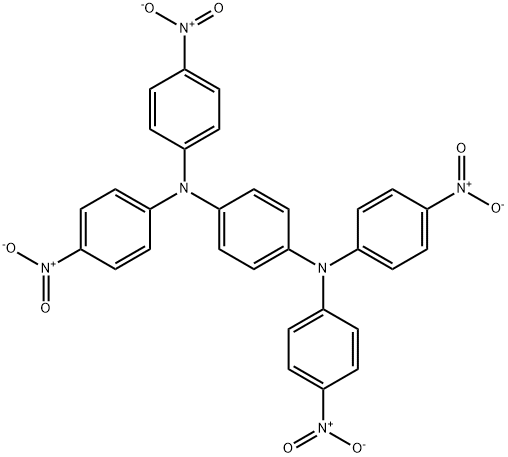
3283-05-4

3283-07-6
Example 4: Preparation of compound (III-1) 160 g of N,N,N',N'-tetrakis(p-nitrophenyl)p-phenylenediamine (II-1), 1.07 g of ferric chloride (III) hexahydrate, 1.55 g of iron (III) oxide, 16.2 g of activated carbon and 1400 ml of 1 -methyl-2-pyrrolidinone were added into a three-necked flask and heated under stirring to an internal temperature of 100 °C. Subsequently, 450 g of 80% hydrazine monohydrate aqueous solution was slowly added dropwise, the reaction temperature was maintained in the range of 100-110 °C, and the reaction was continuously stirred for 5 hours. Upon completion of the reaction, it was cooled to room temperature. The reaction mixture was filtered to remove the activated carbon, followed by the sequential dropwise addition of 800 ml of methanol and 1200 ml of water to the filtrate, and the precipitated crystals were collected by filtration by pumping to afford 126.3 g of N1,N1'-(1,4-phenylene)bis(N1-(4-aminophenyl)benzene-1,4-diamine (III-1) in 99% yield. Spectral analysis of the product showed M/e = 472.

3283-05-4
54 suppliers
$45.00/10g

3283-07-6
124 suppliers
$40.00/1g
Yield:3283-07-6 99%
Reaction Conditions:
with ferric(III) chloride;iron(III) oxide;pyrographite;hydrazine in lithium hydroxide monohydrate;N-methylpyrrolidine N-oxide at 100 - 110; for 6 h;Product distribution / selectivity;
Steps:
4
Example 4: Production of Compound (III-l) 160 g of Compound (II-l), 1.07 g of iron (III) chloride 6-hydrate, 1.55 g of iron (III) oxide, 16.2 g of activated carbon, and 1400 ml of l-methyl-2-pyrrolidone were put into a three-neck flask, and heated with stirring up to an inner temperature of 1000C. 450 g of hydrazine monohydrate (aqueous 80% solution) was dropwise added thereto, taking 1 hour, then this was kept stirred under heat at an inner temperature of from 100 to 1100C for 5 hours, and thereafter cooled to room temperature. This was filtered to remove the activated carbon, then 800 ml of methanol and 1200 ml of water were continuously dropwise added thereto, and the resulting crystal was taken out through suction filtration to obtain 126.3 g of the intended Compound (III-l) (yield, 99%). Its mass spectrum gave M/e = 472.
References:
WO2007/73000,2007,A1 Location in patent:Page/Page column 33