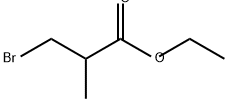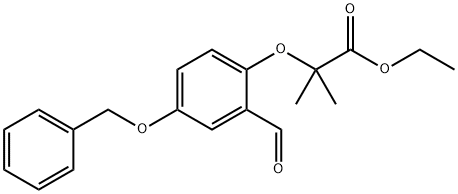
Propanoic acid, 2-[2-forMyl-4-(phenylMethoxy)phenoxy]-2-Methyl-, ethyl ester synthesis
- Product Name:Propanoic acid, 2-[2-forMyl-4-(phenylMethoxy)phenoxy]-2-Methyl-, ethyl ester
- CAS Number:328919-31-9
- Molecular formula:C20H22O5
- Molecular Weight:342.39
Yield:328919-31-9 89%
Reaction Conditions:
with caesium carbonate in DMF (N,N-dimethyl-formamide) at 80; for 18 h;
Steps:
1(A) Preparation 1 2- (4-HYDROXY-2-METHYL-PHENOXY)-2-METHYL-PROPIONIC ACID. Step A 2- (4-BENZVLOXV-2-FORMVL'DHENOXY)-2-METHVL PROPIONIC acid ethyl ester
5-Benzyloxy-2-hydroxy-benzaldehyde (Kappe, T.; Witoszynskyj, T. Arch. Pharm. , 1975,308 (5), 339-346) (2.28 g, 10.0 mmol), ethyl bromoisobutyrate (2.2 mL, 15 mmol), and cesium carbonate (3.26 g, 10.0 mmol) in dry DMF (25 mL) are heated at 80 C for 18 h. The reaction mixture is cooled and partitioned between water (30 mL) and ether (75 mL). The organic layer is washed with brine (15 mL). The aqueous layers are back-extracted with ethyl acetate (30 mL), and the organic layer is washed with brine (20 mL). The combined organic layers are dried (NA2SO4) and concentrated to a brown oil. The crude product is purified by flash chromatography using hexanes: ethyl acetate (2.5 : 1) to give a pale yellow solid (3.04 g, 89%): mp 65 C ;'H NMR (400 MHz, CDC13) 8 1.24 (t, 3H, J = 7.1 Hz), 1.62 (s, 6H), 4. 23 (q, 2H, J = 7.1 Hz), 6.81 (d, 1H, J = 8.8 HZ), 7.10 (dd, 1H, J = 4.6, 9.0 Hz), 7.30-7. 43 (m, 6H); MS (ES) m/e 343.1 [M+1].
References:
WO2004/63155,2004,A1 Location in patent:Page 48-49
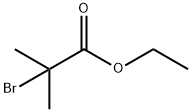
600-00-0
264 suppliers
$13.00/5g
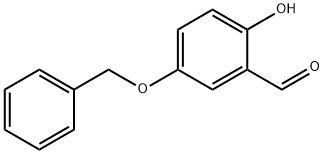
56979-56-7
63 suppliers
$65.00/100mg
![Propanoic acid, 2-[2-forMyl-4-(phenylMethoxy)phenoxy]-2-Methyl-, ethyl ester](/CAS/GIF/328919-31-9.gif)
328919-31-9
5 suppliers
inquiry