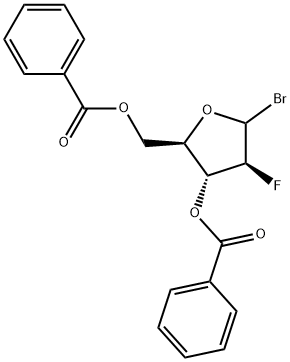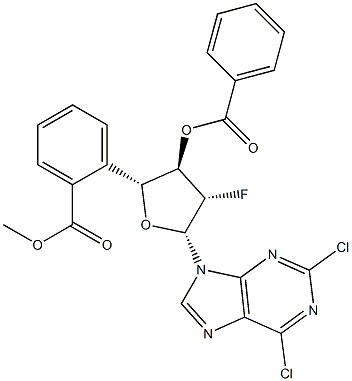
2,6-Dichloropurine -9-beta-D-(2'-deoxy-3',5'-di-O-benzoyl-2'-fluoro)arabinoriboside synthesis
- Product Name:2,6-Dichloropurine -9-beta-D-(2'-deoxy-3',5'-di-O-benzoyl-2'-fluoro)arabinoriboside
- CAS Number:329187-80-6
- Molecular formula:C24H17Cl2FN4O5
- Molecular Weight:531.32
97614-44-3
68 suppliers
$45.00/100mg
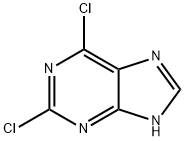
5451-40-1
506 suppliers
$10.00/1g

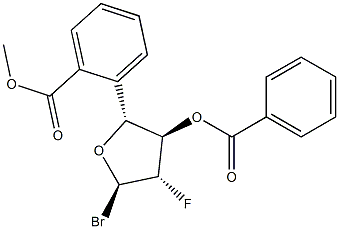
329187-80-6
19 suppliers
inquiry
Yield:329187-80-6 77%
Reaction Conditions:
Stage #1: 2,6 dichloropurinewith caesium carbonate in acetonitrile at 20; for 0.5 h;
Stage #2: 2-deoxy-2-fluoro-3,5-di-O-benzoyl-α-D-arabinofuranosyl bromide in acetonitrile at 20;
Steps:
14.a Step a:
2,6-dichloropurine (3.6 g, 18.8 mmol) was dissolved in 90 mL of acetonitrile and treated with CS2CO3 (7.5 g, 23 mmol, 1.2 equiv.). The mixture was stirred at room temperature for 30 min. The known bromo derivative (8.75 g, 21 mmol, 1.1 equiv.) was dissolved in 100 mL of acetonitrile and added to the mixture dropwise via an addition funnel. The mixture was allowed to stir overnight at room temperature. The mixture was filtered on a pad of silica gel and concentrated. The residue was adsorbed on silica and purified using column chromatography (hexanes / ethyl acetate) to provide the product as a white solid in 77% yield (7.72g). NMR (400 MHz, Chloroform- δ 8.39 (d, J= 3.0 Hz, 1H), 8.10 (ddt, J= 8.5, 3.1, 0.9 Hz, 4H), 7.74 - 7.36 (m, 6H), 6.64 (dd, J = 21.8, 2.8 Hz, 1H), 5.83 - 5.69 (m, 1H), 5.40 (ddd, J = 49.9, 2.8, 0.8 Hz, 1H), 4.89 - 4.77 (m, 2H), 4.62 (q, J = 4.0 Hz, 1H). ESI MS [M+H]+ for C24H17CI2FN4O5, calcd 531.1, found 531.1
References:
WO2018/94148,2018,A1 Location in patent:Paragraph 0214

97614-44-3
68 suppliers
$45.00/100mg

5451-40-1
506 suppliers
$10.00/1g

329187-80-6
19 suppliers
inquiry

5451-40-1
506 suppliers
$10.00/1g

329187-80-6
19 suppliers
inquiry