
7-Hydroxy-2,2-dimethyl-4H-benzo[d][1,3]dioxin-4-one synthesis
- Product Name:7-Hydroxy-2,2-dimethyl-4H-benzo[d][1,3]dioxin-4-one
- CAS Number:330555-87-8
- Molecular formula:C10H10O4
- Molecular Weight:194.18
Yield:330555-87-8 59%
Reaction Conditions:
with trifluoroacetic acid;trifluoroacetic anhydride at 0 - 25; for 72 h;
Steps:
16.1; 17.1; 18.1 Step 1. Synthesis of 7-hydroxy-2, 2-dimethyl-4H-1, 3-benzodioxin-4-one (C50)
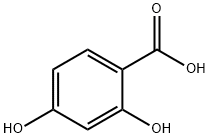
Trifluoroacetic anhydride (300 mL) and acetone (150 mL) were added drop-wise to a 0° C. suspension of 2,4-dihydroxybenzoic acid (55.0 g, 357 mmol) in trifluoroacetic acid (500 mL) and the reaction mixture was stirred at 25° C. for 3 days. Volatiles were removed in vacuo, the residue was added to saturated aqueous sodium bicarbonate solution (500 mL), and the resulting mixture was extracted with ethyl acetate (3×500 mL). The combined organic layers were washed sequentially with water (500 mL) and with saturated aqueous sodium chloride solution (500 mL), dried over sodium sulfate, filtered, and concentrated under reduced pressure. Trituration with dichloromethane (200 mL) provided the product as a white solid. Yield: 41.0 g, 211 mmol, 59%. LCMS m/z 194.7 [M+H]+. 1H NMR (400 MHz, CD3OD) δ 7.73 (d, J=8.5 Hz, 1H), 6.58 (dd, J=8.5, 2.0 Hz, 1H), 6.35 (d, J=2.0 Hz, 1H), 1.69 (s, 6H).
References:
US2018/148432,2018,A1 Location in patent:Paragraph 0363; 0364
89-86-1
537 suppliers
$5.00/10g
![7-Hydroxy-2,2-dimethyl-4H-benzo[d][1,3]dioxin-4-one](/CAS/20180629/GIF/330555-87-8.gif)
330555-87-8
4 suppliers
inquiry
