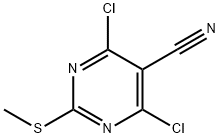
4,6-DICHLORO-2-(METHYLTHIO)PYRIMIDINE-5-CARBONITRILE synthesis
- Product Name:4,6-DICHLORO-2-(METHYLTHIO)PYRIMIDINE-5-CARBONITRILE
- CAS Number:33097-13-1
- Molecular formula:C6H3Cl2N3S
- Molecular Weight:220.08
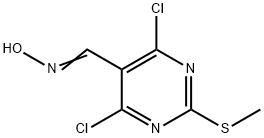
33097-12-0

33097-13-1
General procedure for the synthesis of 2-methylthio-4,6-dichloropyrimidine-5-oxime from 2-methylthio-4,6-dichloro-5-cyanopyrimidine: To 4,6-dichloro-2-(methylthio)-5-pyrimidine carboxaldehyde oxime (2.38 g, 10 mmol) was slowly added dichlorosulfoxide (SOCl2, 21.8 mL, 0.30 mol) at room temperature. Subsequently, the reaction mixture was heated to 75 °C with continuous stirring for about 3 hours. After completion of the reaction, the reaction solution was concentrated under vacuum. The residual dichlorosulfoxide was removed by addition of toluene (5 mL) and evaporated under vacuum. The resulting solid was washed with ethanol/water (10 mL, 1:1 v/v) to afford 4,6-dichloro-2-(methylthio)-5-pyrimidinecarbonitrile (2.04 g, 93% yield). The product was analyzed by LC-MS showing m/z 220 ([M+H]+) with a retention time of 1.99 min; 1H-NMR (CDCl3) δ 2.64 (3H, s).

33097-12-0
21 suppliers
$180.25/1gm:

33097-13-1
152 suppliers
$17.00/250mg
Yield: 93%
Reaction Conditions:
with thionyl chloride at 20 - 75; for 3 h;
Steps:
57.57a
To 4,6-dichloro-2-(methylthio)-5-pyrimidinecarbaldehyde oxime (2.38 g, 10 mmol) was added SOCl2 (21.8 mL, 0.30 mol) slowly at room temperature. The solution was then heated at 75 0C for about 3 hours before it was concentrated under vacuum. The residue SOCl2 was removed by evaporation with toluene (5 mL) under vacuum. The resulting solid was washed with EtOH/H2O (1OmL, 1 : 1) to afford 4,6-dichloro-2-(methylthio)-5-pyrimidinecarbonitrile (2.04 g, 93%). LC-MS m/z 220 (M + H)+1.99 minute; 1H-NMR (CDCl3) δ 2.64(3 H).
References:
GLAXO GROUP LIMITED WO2006/104917, 2006, A2 Location in patent:Page/Page column 81-82
1979-98-2
244 suppliers
$14.00/5g

33097-13-1
152 suppliers
$17.00/250mg
33097-11-9
148 suppliers
$17.00/250mg

33097-13-1
152 suppliers
$17.00/250mg