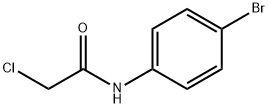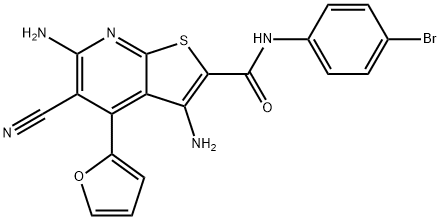
3,6-diamino-N-(4-bromophenyl)-5-cyano-4-(furan-2-yl)thieno[2,3-b]pyridine-2-carboxamide synthesis
- Product Name:3,6-diamino-N-(4-bromophenyl)-5-cyano-4-(furan-2-yl)thieno[2,3-b]pyridine-2-carboxamide
- CAS Number:332046-11-4
- Molecular formula:C19H12BrN5O2S
- Molecular Weight:454.3
Yield:332046-11-4 3.1 g
Reaction Conditions:
Stage #1: 2,6-diamino-4-(furan-2-yl)-4H-thiopyran-3,5-dicarbonitrilewith potassium hydroxide in ethanol;water;N,N-dimethyl-formamide; for 24 h;
Stage #2: N-(4-bromophenyl)-2-chloroacetamide in ethanol;water;N,N-dimethyl-formamide; for 5 h;
Steps:
Compounds 4a-4s (general procedure).
General procedure: An Erlenmeyer flask was charged with a solution of 0.66 g (10 mmol) of malononitrile (1) in 20 mL of ethanol, and three drops of triethylamine were added with stirring using a magnetic stirrer. The mixture was cooled to 10°C on a water bath, and gaseous hydrogen sulfide generated by hydrolysis of Al2S3 was passed through the mixture at a moderate flow rate. The mixture was stirred for 15-20 min after cyanothioacetamide A began to crystallize therefrom, and 10 mmol of aldehyde 2a-2m was added with stirring at room temperature. The mixture became homogeneous for some time, and alkene B separated over a period of 3-8 min. An additional portion of malononitrile (1), 0.66 g (10 mmol), was then added while continuously stirring, and the mixture was stirred for 10-15 min until it became homogeneous again and left to stand for 4-6 h. The mixture was diluted with an equal volume of DMF, 5.6 mL (10 mmol) of 10% aqueous potassium hydroxide was added, and the mixture was left to stand for 24 h. Alkylating agent 3a-3m, 10 mmol, was added, the mixture was stirred for 3 h, and 5.6 mL (10 mmol) of 10% aqueous potassium hydroxide was added again. After 2 h, the mixture was diluted with an equal volume of water, and the precipitate was filtered off and washed with water, ethanol, and hexane.
References:
Dyachenko;Dyachenko;Dorovatovskii;Khrustalev;Nenajdenko [Russian Journal of Organic Chemistry,2018,vol. 54,# 10,p. 1435 - 1445][Zh. Org. Khim.,2018,vol. 54,# 10,p. -1423 - -1433,11]
98-01-1
508 suppliers
$22.00/25g
![3,6-diamino-N-(4-bromophenyl)-5-cyano-4-(furan-2-yl)thieno[2,3-b]pyridine-2-carboxamide](/CAS/20210111/GIF/332046-11-4.gif)
332046-11-4
5 suppliers
inquiry