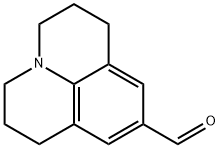
2,3,6,7-Tetrahydro-1H,5H-benzo[ij]quinolizine-9-carboxaldehyde synthesis
- Product Name:2,3,6,7-Tetrahydro-1H,5H-benzo[ij]quinolizine-9-carboxaldehyde
- CAS Number:33985-71-6
- Molecular formula:C13H15NO
- Molecular Weight:201.26
479-59-4

68-12-2
![2,3,6,7-Tetrahydro-1H,5H-benzo[ij]quinolizine-9-carboxaldehyde](/CAS/GIF/33985-71-6.gif)
33985-71-6
Julolidine (0.5 g, 2.89 mmol), N,N-dimethylformamide (0.255 g, 3.49 mmol), and phosphorous trichloride (0.535 g, 3.49 mmol) were dissolved in dichloromethane (15 mL), and the mixture was stirred for 4 h at room temperature under argon protection. The color of the solution changed to green during the reaction and the progress of the reaction was monitored by thin layer chromatography (TLC). Upon completion of the reaction, the reaction was quenched with water and the crude product was subsequently extracted with 2 M sodium hydroxide solution and ether. The organic phase was washed twice with water, dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by column chromatography with 40%-50% ether/hexane as eluent to give 0.48 g (83.08% yield) of 1,2,3,5,6,7-hexahydropyrido[3,2,1-ij]quinoline-9-carboxaldehyde as a light-yellow solid (up to 92% when 1.5 g of julonidine was used). The structure of the product was confirmed by 1H NMR (300 MHz, CDCl3): δ 9.597 (s, 1H), 7.29 (s, 1H), 3.308 (t, J = 57 Hz, 4H), 2.787 (t, J = 62 Hz, 4H), 2.002-1.92 (m, J = 63 Hz, 4H).