
4-FLUORO-N-([3-(4-METHOXYPHENYL)-5-ISOXAZOLYL]METHYL)BENZENESULFONAMIDE synthesis
- Product Name:4-FLUORO-N-([3-(4-METHOXYPHENYL)-5-ISOXAZOLYL]METHYL)BENZENESULFONAMIDE
- CAS Number:343372-73-6
- Molecular formula:C17H15FN2O4S
- Molecular Weight:362.38
![1-[3-(4-methoxyphenyl)-5-isoxazolyl]methanamine(SALTDATA: FREE)](/CAS/GIF/338982-43-7.gif)
338982-43-7
22 suppliers
$90.00/100mg
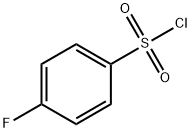
349-88-2
314 suppliers
$6.00/5g
![4-FLUORO-N-([3-(4-METHOXYPHENYL)-5-ISOXAZOLYL]METHYL)BENZENESULFONAMIDE](/StructureFile/ChemBookStructure4/GIF/CB1490909.gif)
343372-73-6
5 suppliers
inquiry
Yield:343372-73-6 20%
Reaction Conditions:
with pyridine in dichloromethane at 25; for 1.33333 h;
Steps:
2.3. Synthesis of isoxazolyl-sulfonamide derivatives (1-20)
General procedure: The condensation between 3-phenyl-5-hydroxyamine-isoxazoles derivatives (compound d) andfour benzenesulfonyl chlorides (4-nitro, 4-flour, 4-chloro and 4-methyl, 1.5 eq) was executed at 25 °C,in freshly distilled CH2Cl2, in presence of pyridine (1.5 eq). After 80 min, the reaction was diluted withCH2Cl2 and this organic phase was washed with HCl(aq) 0.1 M, to remove unreacted amine and tracesof pyridine; and NaCl(aq). The synthesized compounds were purified by column chromatography on SiO2using hexane and EtOAc [6:4 (v/v)] as eluent. Procedure adapted from Schwarz et al2.
References:
da Rosa, Rafael;Zimmermann, Lara Almida;de Moraes, Milene H?ehr;Schneider, Naira Fernanda Zanchett;Schappo, Alice Duarte;Sim?es, Claudia Maria de Oliveira;Steindel, Mario;Schenkel, Eloir Paulo;Bernardes, Lílian Sibelle Campos [Bioorganic and Medicinal Chemistry Letters,2018,vol. 28,# 20,p. 3381 - 3384] Location in patent:supporting information
1262439-59-7
0 suppliers
inquiry
![4-FLUORO-N-([3-(4-METHOXYPHENYL)-5-ISOXAZOLYL]METHYL)BENZENESULFONAMIDE](/StructureFile/ChemBookStructure4/GIF/CB1490909.gif)
343372-73-6
5 suppliers
inquiry

325744-41-0
43 suppliers
$54.00/100mg
![4-FLUORO-N-([3-(4-METHOXYPHENYL)-5-ISOXAZOLYL]METHYL)BENZENESULFONAMIDE](/StructureFile/ChemBookStructure4/GIF/CB1490909.gif)
343372-73-6
5 suppliers
inquiry
![[3-(4-METHOXY-PHENYL)-ISOXAZOL-5-YL]-METHANOL](/CAS/GIF/206055-86-9.gif)
206055-86-9
80 suppliers
$21.00/250mg
![4-FLUORO-N-([3-(4-METHOXYPHENYL)-5-ISOXAZOLYL]METHYL)BENZENESULFONAMIDE](/StructureFile/ChemBookStructure4/GIF/CB1490909.gif)
343372-73-6
5 suppliers
inquiry