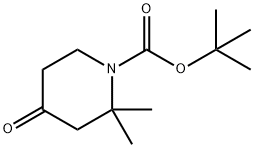
2,2-DiMethyl-4-oxopiperidine-1-carboxylic acid tert-butyl ester synthesis
- Product Name:2,2-DiMethyl-4-oxopiperidine-1-carboxylic acid tert-butyl ester
- CAS Number:346593-03-1
- Molecular formula:C12H21NO3
- Molecular Weight:227.3

79099-07-3

74-88-4

346593-03-1
GENERAL STEPS: N-tert-butoxycarbonyl-4-piperidone (5 g, 25 mmol) was dissolved in tetrahydrofuran (100 mL) and the resulting solution was cooled to 0 °C. Under cooling conditions, sodium hydride (60% dispersed in mineral oil, 2.10 g, 53 mmol) was added all at once and the resulting turbid mixture was stirred for 10 minutes. Subsequently, iodomethane was added slowly and the reaction mixture was gradually warmed up to room temperature with continuous stirring overnight (12 hours). Upon completion of the reaction, the light orange colored mixture was concentrated under reduced pressure. The concentrated residue was extracted by partitioning with ether and water. The aqueous phase was back-extracted with ether and all organic phases were combined, washed sequentially with water and saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated to give a light yellow solid. The solid was ground with a hexane solution of 4% ethyl acetate (50 mL) to afford 1-Boc-2,2-dimethylpiperidin-4-one as a cream-colored solid (1.8 g, 32% yield). The product was characterized by 1H-NMR (CDCl3, 300 MHz): δ 3.73 (t, 2H), 3.43 (br s, 2H), 2.49 (t, 2H), 1.49 (s, 9H), 1.13 (s, 6H).

79099-07-3
0 suppliers
$5.00/5g

74-88-4
357 suppliers
$15.00/10g

346593-03-1
91 suppliers
$84.00/200 μmol
Yield:346593-03-1 32%
Reaction Conditions:
Stage #1:N-tert-butyloxycarbonylpiperidin-4-one with sodium hydride in tetrahydrofuran at 0; for 0.166667 h;
Stage #2:methyl iodide in tetrahydrofuran at 0 - 20;
Steps:
80
4-Oxo-piperidine-1-carboxylic acid tert-butyl ester (5 g, 25 mmol) was dissolved in tetrahydrofuran (100 mL) and the resulting solution was cooled to 0° C. Sodium hydride (60% in mineral oil, 2.10 g, 53 mmol) was added to the cooled solution in a single portion, and the resulting cloudy mixture was allowed to stir 10 min. Methyl iodide was subsequently added and the mixture was allowed to warm to room temperature over several hours. Stirring continued over night (12 h). The light orange mixture was concentrated under reduced pressure. The residue was partitioned between ether and water. The aqueous phase was back-extracted with additional ether. The combined extracts were washed with water and brine, dried over sodium sulfate, filtered and concentrated to a pale yellow solid. The solid was triturated with 4% ethyl acetate in hexanes (50 mL) to afford 3,3-dimethyl-4-oxo-piperidine-1-carboxylic acid tert-butyl ester as a cream-colored solid (1.8 g, 32%). 1H-NMR (CDCl3, 300 MHz) δ 3.73 (t, 2H), 3.43 (br s, 2H), 2.49 (t, 2H), 1.49 (s, 9H), 1.13(s, 6H).
References:
Millennium Pharmaceuticals, Inc. US2005/239781, 2005, A1 Location in patent:Page/Page column 110