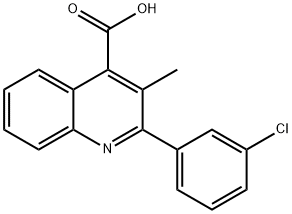
2-(3-CHLORO-PHENYL)-3-METHYL-QUINOLINE-4-CARBOXYLIC ACID synthesis
- Product Name:2-(3-CHLORO-PHENYL)-3-METHYL-QUINOLINE-4-CARBOXYLIC ACID
- CAS Number:350997-46-5
- Molecular formula:C17H12ClNO2
- Molecular Weight:297.74
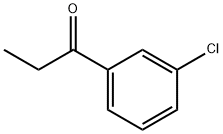
34841-35-5
485 suppliers
$5.00/5g
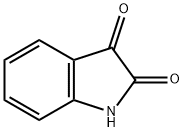
91-56-5
473 suppliers
$5.00/25g

350997-46-5
27 suppliers
$57.50/250mg
Yield:350997-46-5 99%
Reaction Conditions:
Stage #1: indole-2,3-dionewith potassium hydroxide in ethanol;water; for 0.25 h;
Stage #2: 3'-chloro-propiophenone in ethanol;water; for 18 h;Reflux;
Stage #3: with hydrogenchloride in water;Cooling with ice;
Steps:
4
EXAMPLE 43-(1 ,4'-bipiperidin-1 '-ylmethyl)-2-(3-chlorophenyl)-//-(1-phenylcvclopropyl)-4-2-(3-chlorophenyl)-3-methyl-4-quinolinecarboxylic acidPotassium hydroxide (1 1.44 g, 203.9 mmol) in water (22 mL) was added dropwise to a suspension of 1 H-indole-2,3-dione (5 g, 34 mmol) in ethanol (60 mL) over a 15 min period. 1-(3-Chlorophenyl)-1 -propanone (5.73 g, 34 mmol) was added and the resulting mixture was heated to reflux for 18 h. The reaction mixture was then cooled to room temperature and the solvent was removed in vacuo. The residue was diluted with water, washed with diethyl ether, cooled in an ice bath, and acidified with 1 N HCI. The precipitate was collected by filtration, washed with water, and dried to afford 2-(3-chlorophenyl)-3-methyl-4- quinolinecarboxylic acid (10 g, 99% yield) as a pale yellow solid. MS (m/z) 298.0 (M+H+).
References:
WO2011/119704,2011,A1 Location in patent:Page/Page column 54