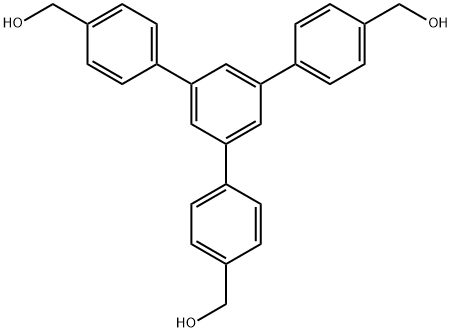
[1,1',3',1",3",1"'-Quaterphenyl]-3,3'''-dimethylalcohol synthesis
- Product Name:[1,1',3',1",3",1"'-Quaterphenyl]-3,3'''-dimethylalcohol
- CAS Number:353289-47-1
- Molecular formula:C27H24O3
- Molecular Weight:396.48
![1,3,5-tris[(4-methoxycarbonyl)phenyl]benzene](/CAS/20180703/GIF/117100-41-1.gif)
117100-41-1
8 suppliers
inquiry

353289-47-1
30 suppliers
inquiry
Yield: 82%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 30; for 24 h;Inert atmosphere;
Steps:
1,3,5-Tris[(4-hydroxymethyl)phenyl]benzene (5)
A lithium aluminum hydride (0.19 g, 5.0 mmol) was addedto 20 mL of dry THF. Then solution of 4 (0.4 g, 0.83 mmol) in 20 mL of dry THF was added dropwise at 0 C under stirring and an atmosphere of argon. After dropwise addition was completed, the mixture was heated to 30 C for 24 h.The excess of reducing agent was destroyed by slow addition of 5 % HCl (5 mL) and the solvent was evaporated. Reaction mixture was added to water (400 mL) and extracted with chloroform (4 9 300 mL). The organic layers were combined and removed under reduced pressure. The crude material was purified through a column chromatography of silica gel with a 50:1 mixture of chloroform: methanol as aneluent. The solvent was evaporated under high vacuum to afford 0.27 g (82 % yield) of 5. 1H NMR (300 MHz,DMSO-d6): d = 7.85 (s, 3H), 7.82 (d, J = 8.21 Hz, 6H),7.45(d, J = 8.25 Hz, 6H), 5.23 (t, J = 5.72 Hz, 3H), 4.57(d, J = 5.68, 6H) ppm. 13C NMR (75.4 MHz, DMSO-d6): d = 142.08, 141.48, 138.51, 126.99, 126.82, 123.89,62.62 ppm. MS (ESI): m/z 396.1. The analytical data areconsistent with those in the literature [22].
References:
Bednaříková, Tereza;Tosňer, Zdeněk;Horský, Jiří;Jindřich, Jindřich [Journal of Inclusion Phenomena and Macrocyclic Chemistry,2015,vol. 81,# 1,p. 141 - 152]
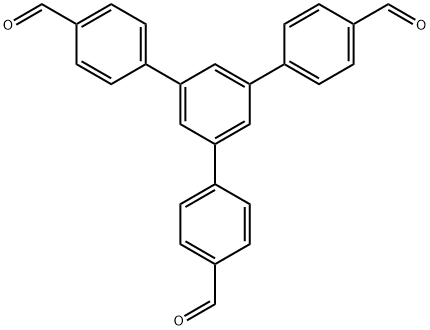
118688-53-2
113 suppliers
$28.00/250mg

353289-47-1
30 suppliers
inquiry
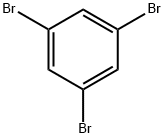
626-39-1
478 suppliers
$5.00/5g

353289-47-1
30 suppliers
inquiry
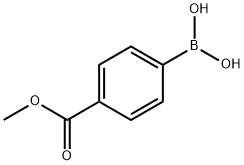
99768-12-4
372 suppliers
$10.00/1 g

353289-47-1
30 suppliers
inquiry