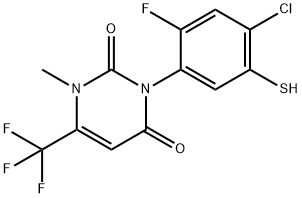
3-(4-chloro-2-fluoro-5-sulfanylphenyl)-1-methyl-6-(trifluoromethyl)-1,2,3,4-tetrahydropyrimidine-2,4-dione synthesis
- Product Name:3-(4-chloro-2-fluoro-5-sulfanylphenyl)-1-methyl-6-(trifluoromethyl)-1,2,3,4-tetrahydropyrimidine-2,4-dione
- CAS Number:353292-92-9
- Molecular formula:C12H7ClF4N2O2S
- Molecular Weight:354.71

114136-66-2
6 suppliers
inquiry

353292-92-9
17 suppliers
inquiry
Yield:353292-92-9 58%
Reaction Conditions:
Stage #1: 1-methyl-6-trifluoromethyl-3-(4-chloro-2-fluorophenyl)-2,4(1H,3H)-pyrimidinedionewith sulfonic chloride acid at 130 - 135;
Stage #2: with hydrogenchloride;stannous chloride dihydrate;glacial acetic acid at 80; for 5 h;
Steps:
Scheme 2. Synthesis of pyrimidinedione derivatives 10
Amass of 14.79 g (126.92 mmol) of chlorosulfonic acid wasplaced in a round bottom flask and the internal temperaturewas adjusted within the range of -10 C to 5 C. It wasfollowed by the addition of 4.10 g (12.69 mmol) of 3-(4-chloro-2-fluorophenyl)-1-methyl-6-(trifloromethyl)pyrimidine-2,4(1H,3H)-dione (7). The reaction was kept for 3 h at130-135C. When the reaction was completed, ice-coldwater was added to terminate the reaction. It was extractedtwice with EtOAc, dried with anhydrous MgSO4, filtered, and concentrated under reduced pressure. The crude mixtureobtained was then diluted in 38 mL acetic acid(AcOH). It was followed by the addition of 14.37 mL conc.HCl and 14.32 g (63.46 mmol) tin(II) chloride dihydrate(SnCl22H2O). After 5 h of reaction at 80C, H2O wasadded and the reaction mixture was extracted twice withmethylene chloride (CH2Cl2). The organic layer was driedwith anhydrous MgSO4, filtered, and concentrated underreduced pressure. The crude mixture was purified usingflash column chromatography which obtained 2.61 g(7.36 mmol, 58% yield) of compound 8 as a white solid.
References:
Choi, Jong-Soo;Kwon, Young Bin;Lee, Kye Hwan;Lee, Won Hyung;Seu, Young-Bae [Bulletin of the Korean Chemical Society,2021,vol. 42,# 3,p. 420 - 428]

114136-63-9
2 suppliers
inquiry

353292-92-9
17 suppliers
inquiry
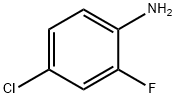
57946-56-2
352 suppliers
$12.00/25g

353292-92-9
17 suppliers
inquiry

69922-26-5
6 suppliers
inquiry

353292-92-9
17 suppliers
inquiry
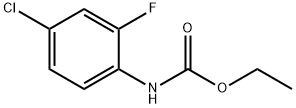
114108-90-6
10 suppliers
inquiry

353292-92-9
17 suppliers
inquiry