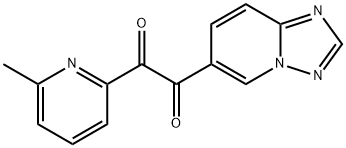
1-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-2-(6-methylpyridin-2-yl)ethane-1,2-dione synthesis
- Product Name:1-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-2-(6-methylpyridin-2-yl)ethane-1,2-dione
- CAS Number:356560-84-4
- Molecular formula:C14H10N4O2
- Molecular Weight:266.25
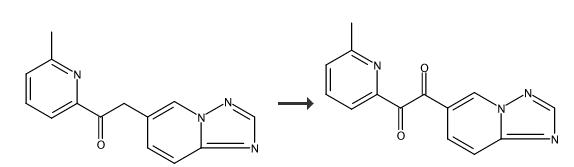
Preparation of 1-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-2-(6-methylpyridin-2-yl)ethane-1,2-dione (a compound of the formula ( V) wherein Ra = CH3) To a stirred suspension of 2-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-1-(6-methylpyridin-2-yl)ethanone (6.20 g, 24.57 mmol) in DMSO (48 mL) was added dropwise HBr (48 wt. % in water, 5.96 g, 12.4 mL) at 0°C, and the mixture was heated at 60-70°C. After 2 h, the reaction mixture was cooled to 0°C, poured onto ice water (20 mL), and basified to pH 10 with solid K2CO3. The mixture was extracted with CHCl3 (2 x 250 mL), and the organic phase was washed with water (2 x 100 mL), dried over anhydrous Na2SO4, filtered, and evaporated to dryness under reduced pressure. The residue was purified by MPLC on silica gel using a mixture of MeOH and CH2Cl2 as eluent to give the titled compound. Light yellow solid, yield 6.02 g, 92%
![Ethanone, 1-(6-Methyl-2-pyridinyl)-2-[1,2,4]triazolo[1,5-a]pyridin-6-yl-](/CAS/GIF/614750-82-2.gif)
614750-82-2
26 suppliers
inquiry
![1-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-2-(6-methylpyridin-2-yl)ethane-1,2-dione](/CAS/GIF/356560-84-4.gif)
356560-84-4
71 suppliers
$174.00/100mg
Yield:356560-84-4 92%
Reaction Conditions:
with hydrogen bromide;dimethyl sulfoxide at 60 - 70; for 2 h;
Steps:
5
To a stirred suspension of 2-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-1-(6-methylpyridin-2-yl)ethanone (6.20 g, 24.57 mmol) in DMSO (48 mL) was added dropwise HBr (48 wt. % in water, 5.96 g, 12.4 mL) at 0° C., and the mixture was heated at 60-70° C. After 2 h, the reaction mixture was cooled to 0° C., poured onto ice water (20 mL), and basified to pH 10 with solid K2CO3. The mixture was extracted with CHCl3 (2×250 mL), and the organic phase was washed with water (2×100 mL), dried over anhydrous Na2SO4, filtered, and evaporated to dryness under reduced pressure. The residue was purified by MPLC on silica gel using a mixture of MeOH and CH2Cl2 as eluent to give the titled compound (6.02 g, 92%) as a light yellow solid. 1H NMR (400 MHz, CDCl3): δ 9.11 (dd, 1H, J=1.6, 1.2 Hz), 8.47 (s, 1H), 8.14 (dd, 1H, J=9.2, 1.6 Hz), 8.04 (br d, 1H, J=7.6 Hz), 7.88 (dd, 1H, J=9.2, 1.2 Hz), 7.84 (t, 1H, J=7.8 Hz), 7.42 (br d, 1H, J=8.0 Hz), 2.49 (s, 3H).
References:
US8080568,2011,B1 Location in patent:Page/Page column 19
![[1,2,4]Triazolo[1,5-a]pyridine-6-carboxaldehyde (9CI)](/CAS/GIF/614750-81-1.gif)
614750-81-1
109 suppliers
$68.00/100mg
![1-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-2-(6-methylpyridin-2-yl)ethane-1,2-dione](/CAS/GIF/356560-84-4.gif)
356560-84-4
71 suppliers
$174.00/100mg
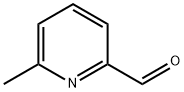
1122-72-1
302 suppliers
$7.00/1g
![1-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-2-(6-methylpyridin-2-yl)ethane-1,2-dione](/CAS/GIF/356560-84-4.gif)
356560-84-4
71 suppliers
$174.00/100mg
![Phosphonic acid, P-[(6-Methyl-2-pyridinyl)(phenylaMino)Methyl]-, diphenyl ester](/CAS/GIF/614750-85-5.gif)
614750-85-5
21 suppliers
inquiry
![1-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-2-(6-methylpyridin-2-yl)ethane-1,2-dione](/CAS/GIF/356560-84-4.gif)
356560-84-4
71 suppliers
$174.00/100mg