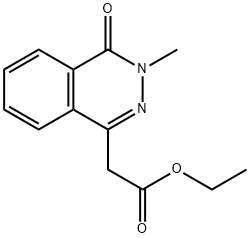
Ethyl 2-(3-Methyl-4-Oxo-3,4-Dihydrophthalazin-1-Yl)Acetate synthesis
- Product Name:Ethyl 2-(3-Methyl-4-Oxo-3,4-Dihydrophthalazin-1-Yl)Acetate
- CAS Number:356790-62-0
- Molecular formula:C13H14N2O3
- Molecular Weight:246.26
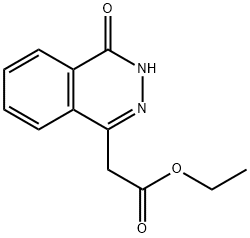
25947-13-1
32 suppliers
$172.00/250mg

74-88-4
356 suppliers
$15.00/10g

356790-62-0
16 suppliers
$354.80/250mg
Yield:356790-62-0 95%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20; for 3 h;
Steps:
Procedure for N-alkylation of ethyl 2-(3-methyl-4-oxo-3,4-dihydrophthalazin-l- yl)acetate
Procedure for N-alkylation of ethyl 2-(3-methyl-4-oxo-3,4-dihydrophthalazin-l- yl)acetate
To a suspension of dry K2CO3 (7.77 g, 55.5 mmol, 3 eq) in DMF (80 mL) is added phthalazinone (4.23 g, 18.5 mmol, 1 eq). Methyl iodide is then added (1 1 mL, 175 mmol, 9.5 eq). Once completion of the addition, stirring is maintained for 3 hours at 20°C then the mixture is quenched with a saturated solution of NH4CI (200 mL). The product is extracted with AcOEt. The organic layers are washed with water then brine, drying over MgS04 and concentrated in vaccuo to give a solid residue which is purified by column chromatography on silica gel (eluent : PE/AcOEt ; 8/2). (0176) 15 (0177) (0178) 12 (0179) Chemical Formula: C 3H 4N2 (0180) Molecular Weight: 246.27 (0181) Yield : 95 %, white solid (0182) Rf : 0.26 (PE/Ethyl Acetate, 8/2) 1H NMR (400 MHz in CDCI3) : δ (ppm) 8.47 (m, 1H, H-6) ; 7,82-7.68 (m, 3H, H-3, H-2, H- 1) ; 4.20 (q, 3J = 7.2, 2H, H-l l) ; 3.96 (s, 2H, H-l l) ; 3.84 (s, 3H, H-18) ; 1.24 (t, 3H, 3J = 7.2, H-17). (0183) 13C NMR (100 MHz in CDCI3) : δ (ppm) 169.9 (C-13) ; 159.7 (C-7) ; 140.4 (C-10) ; 133.1 (C-l) ; 131.1 (C-2) ; 129.6 (C-5) ; 128.0 (C-4) ; 127.3 (C-3) ; 124.6 (C-6) ; 61.6 (C- 16) ; 39.5 (C-l 8) ; 39.1 (C-l l) ; 14.3 (C-17). (0184) HRMS-MALDI : calcd for C13H15N203 [M+H]+ : 247.1077, found : 247.1086. (0185) Mp : 86.3 °C (toluene)
References:
WO2015/181394,2015,A1 Location in patent:Page/Page column 18
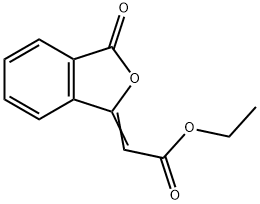
13141-32-7
0 suppliers
inquiry
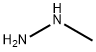
60-34-4
0 suppliers
$65.59/25g

356790-62-0
16 suppliers
$354.80/250mg
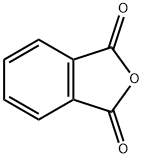
85-44-9
780 suppliers
$9.00/5g

356790-62-0
16 suppliers
$354.80/250mg
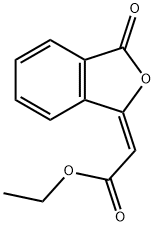
57691-07-3
3 suppliers
inquiry

356790-62-0
16 suppliers
$354.80/250mg

57691-07-3
3 suppliers
inquiry

60-34-4
0 suppliers
$65.59/25g

356790-62-0
16 suppliers
$354.80/250mg