
1-(4-CYCLOPENTYLAMINO-2-METHYLSULFANYL-PYRIMIDIN-5-YL)-ETHANONE synthesis
- Product Name:1-(4-CYCLOPENTYLAMINO-2-METHYLSULFANYL-PYRIMIDIN-5-YL)-ETHANONE
- CAS Number:362656-11-9
- Molecular formula:C12H17N3OS
- Molecular Weight:251.35
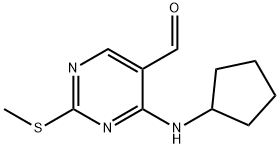
211245-64-6
34 suppliers
$356.00/1g

75-16-1
274 suppliers
$12.00/10ml

362656-11-9
17 suppliers
inquiry
Yield:362656-11-9 88%
Reaction Conditions:
Stage #1: 4-cyclopentylamino-2-methylsulfanyl-pyrimidine-5-carbaldehyde;methylmagnesium bromide in tetrahydrofuran;diethyl ether; for 1 h;Inert atmosphere;Cooling with ice;
Stage #2: with manganese(IV) oxide in chloroform; for 22 h;Reflux;
Steps:
1 Conversion of Compound D to Compound E
(0128) Compound D (40 g, 169 mmol) was dissolved in anhydrous THF (800 mL) under nitrogen and the solution was cooled in ice bath, to which MeMgBr was added slowly (160 mL, 480 mmol, 3 M in ether) and stirred for 1 h. The reaction was quenched with saturated aqueous NH4Cl the partitioned between water and EtOAc. The organic layer was separated and the aqueous layer was extracted with EtOAc. The combined organic were washed with brine and dried over MgSO4. Concentration gave an intermediate product as an oil (41.9 g, 98%). (0129) The above intermediate (40 g, 158 mmol) was dissolved in dry CHCl3 (700 mL). MnO2(96 g, 1.11 mol) was added and the mixture was heated to reflux with stirring for 18 h and another MnO2 (34 g, 395 mmol) was added and continue to reflux for 4 h. The solid was filtrated through a Celite pad and washed with CHCl3. The filtrate was concentrated to give a yellow solid compound E (35 g, 88%), Mp: 75.8-76.6°C.
References:
EP2429566,2016,B1 Location in patent:Paragraph 127-129

362656-31-3
6 suppliers
inquiry

362656-11-9
17 suppliers
inquiry
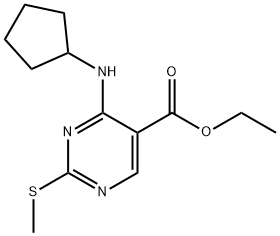
211245-62-4
45 suppliers
inquiry

362656-11-9
17 suppliers
inquiry
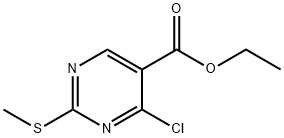
5909-24-0
418 suppliers
$6.00/5g

362656-11-9
17 suppliers
inquiry