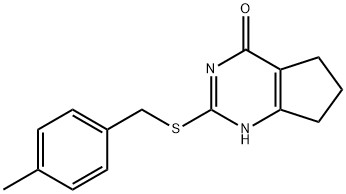
2-((4-methylbenzyl)thio)-3,5,6,7-tetrahydro-4H-cyclopenta[d]pyrimidin-4-one synthesis
- Product Name:2-((4-methylbenzyl)thio)-3,5,6,7-tetrahydro-4H-cyclopenta[d]pyrimidin-4-one
- CAS Number:370847-39-5
- Molecular formula:C15H16N2OS
- Molecular Weight:272.37
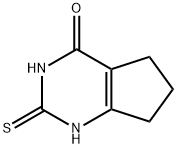
35563-27-0
39 suppliers
$141.00/250mg
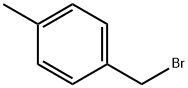
104-81-4
207 suppliers
$17.00/5g
![2-((4-methylbenzyl)thio)-3,5,6,7-tetrahydro-4H-cyclopenta[d]pyrimidin-4-one](/CAS/20180527/GIF/370847-39-5.gif)
370847-39-5
4 suppliers
inquiry
Yield:370847-39-5 92%
Reaction Conditions:
with potassium carbonate in acetone; for 12 h;Reflux;
Steps:
(1) General Procedure for Synthesis of MMP-13 Inhibitors 4, 20, and 24
5,6-Trimethylene-2-thiouracil (A) was synthesized following a literature procedure.1 A mixture of 0.5 mmol A, 0.5 mmol p-substituted benzyl bromide, and 1.5 mmol potassium carbonate in 3 mL of acetone was heated at reflux for 12 h. The reaction was allowed to cool to ambient temperature, and the resulted slurry was diluted with water and extracted with methylene chloride. The organic layer was diluted with methanol (to dissolve residual solids), dried over magnesium sulfate and then filtered. Silica gel was added to the filtrate, which was then concentrated in vacuo. The resulted solid was loaded onto the top of a silica gel column and eluted with 1% to 5% methanol in methylene chloride to afford the targeted MMP-13 inhibitors.
References:
Roth, Joshua;Minond, Dmitriy;Darout, Etzer;Liu, Qin;Lauer, Janelle;Hodder, Peter;Fields, Gregg B.;Roush, William R. [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 23,p. 7180 - 7184] Location in patent:supporting information; experimental part
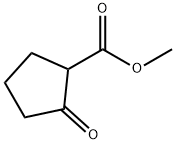
10472-24-9
457 suppliers
$6.00/10g
![2-((4-methylbenzyl)thio)-3,5,6,7-tetrahydro-4H-cyclopenta[d]pyrimidin-4-one](/CAS/20180527/GIF/370847-39-5.gif)
370847-39-5
4 suppliers
inquiry