
Cyclobutanecarboxaldehyde, 2-[(acetyloxy)methyl]-, (1R,2R)- (9CI) synthesis
- Product Name:Cyclobutanecarboxaldehyde, 2-[(acetyloxy)methyl]-, (1R,2R)- (9CI)
- CAS Number:371784-53-1
- Molecular formula:C8H12O3
- Molecular Weight:156.1791
![[(1R,2S)-2-(Hydroxymethyl)cyclobutyl]methyl Acetate](/CAS/20200401/GIF/98516-06-4.gif)
98516-06-4
12 suppliers
inquiry
![Cyclobutanecarboxaldehyde, 2-[(acetyloxy)methyl]-, (1R,2R)- (9CI)](/CAS/GIF/371784-53-1.gif)
371784-53-1
8 suppliers
inquiry
Yield:371784-53-1 88%
Reaction Conditions:
Stage #1: ((1R,2S)-2-(hydroxymethyl)cyclobutyl)methyl acetatewith oxalyl dichloride;dimethyl sulfoxide;triethylamine in dichloromethane at -78;Swern Oxidation;
Stage #2: with triethylamine in dichloromethane at 25; for 120 h;
Steps:
b
[0049] The syntheses of the C12-C13-cyclobutyl aldehydes 15 and 16 were carried out as shown in Scheme 4. As these compounds are very closely related to the cyclopropane derivatives 13 and 14, a similar synthetic route was again followed. Thus, starting from the monoacetate 33, readily available through enzymatic group-selective saponification of the corresponding diacetate (Laumen, K.; Schneider, M. Tetrahedron Lett. 1985, 26, 2073-2076; Kasel, W.; et al. J. Chem. Soc., Chem. Commun. 1985, 1563-1564), cis-aldehyde 34 was prepared by Dess-Martin periodinane oxidation (95% yield), while the corresponding trans-aldehyde 39 was conveniently available by base-catalyzed epimerization of 34 (88% from 33). Following the route described for the cyclopropyl derivatives, 34 and 39 were homologated to 35 and 40, respectively, and the latter compounds were coupled with the chiral phosphorane derived from enantiomerically pure phosphonium salt 21 and NaHMDS-TMSCI to yield olefins 36 and 41, respectively. Hydrogenation of the double bond using a platinum catalyst, followed by standard protecting group manipulations, afforded alcohols 38 and 43, which were again homologated and protected, as summarized in Scheme 4, thus producing aldehydes 15 and 16, respectively; [0086] FIG. 5 is a scheme for the synthesis of building block aldehydes 15 and 16. Reagents and Conditions: (a) DMP (1.2 equiv), NaHCO3 (5.0 equiv), CH2Cl2, 25° C., 3 h, 95%; (b) starting with alcohol 33: (COCl)2 (1.1 equiv), DMSO (2.2 equiv), Et3N (5.0 equiv), CH2Cl2, -78° C.; then Et3N, 25° C., 5 days, 88% over 2 steps; c) MeOCH2PPh3Cl (1.15 equiv), NaHMDS (1.10 equiv), THF, -78 to 25° C., 89%; (d) 0.12 N HCl (aq):acetone (1:9), reflux, 1 h, 98% (35), 94% (40); (e) 21 (2.0 equiv), NaHMDS (3.8 equiv), THF, 0° C., 2 h; then TMSCI (2.0 equiv), 25° C., 20 min; then 35 (or 40), THF, -78 to 25° C., 20 h, 59% (36), 83% (41); (f) (NCO2K)2 (20 equiv), AcOH (40 equiv), py:MeOH (5:1), 25° C., 48 h; then PtO2 (0.05 equiv), H2 (1 atm), MeOH, 25° C., 20 min, 82%; (g) 10 wt % Pt on carbon (0.02 equiv), EtOAc, 25° C., 8 h, 96%; (h) TBSOTf (1.0 equiv), 2,6-lutidine (2.5 equiv), CH2Cl2, -78 to 0° C., 20 min; (i) DIBAL (2.0 equiv), CH2Cl2, -78° C., 5 min, 99% (38), 90% (43) for 2 steps; (j) DMP (1.2 equiv), NaHCO3 (5.1 equiv), CH2Cl2, 25° C., 3 h, 94%; (k) (COCl)2 (1.1 equiv), DMSO (2.2 equiv), Et3N (5.0 equiv), CH2Cl2, -78 to 25° C., 97%; (l) MeOCH2PPh3Cl (1.15 equiv), NaHMDS (1.10 equiv), THF, -78 to 25° C.; (m) 0.12 N HCl (aq):acetone (1:9), reflux, 1 h; (n) Ac2O (1.1 equiv), Et3N (2.5 equiv), 4-DMAP (0.02 equiv), CH2Cl2, 0° C., 20 min, 60% (15), 62% (16) for 3 steps. DIBAL=diisobutylaluminum hydride, 4-DMAP=4-dimethylamino-pyridine, DMP=Dess-Martin periodinane, NaHMDS=sodium hexamethyldisilazide, py=pyridine, TMSCI=chlorotrimethylsilane.
References:
US2004/39026,2004,A1 Location in patent:Page/Page column 6; 10-11; 5/16
![Cyclobutanecarboxaldehyde, 2-[(acetyloxy)methyl]-, (1S,2R)- (9CI)](/CAS/GIF/329010-17-5.gif)
329010-17-5
1 suppliers
inquiry
![Cyclobutanecarboxaldehyde, 2-[(acetyloxy)methyl]-, (1R,2R)- (9CI)](/CAS/GIF/371784-53-1.gif)
371784-53-1
8 suppliers
inquiry
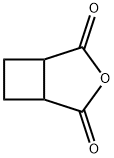
4462-96-8
125 suppliers
$23.00/250mg
![Cyclobutanecarboxaldehyde, 2-[(acetyloxy)methyl]-, (1R,2R)- (9CI)](/CAS/GIF/371784-53-1.gif)
371784-53-1
8 suppliers
inquiry
![[(1R,2S)-rel-2-[(acetyloxy)methyl]cyclobutyl]methyl acetate](/CAS/20211123/GIF/98515-98-1.gif)
98515-98-1
12 suppliers
inquiry
![Cyclobutanecarboxaldehyde, 2-[(acetyloxy)methyl]-, (1R,2R)- (9CI)](/CAS/GIF/371784-53-1.gif)
371784-53-1
8 suppliers
inquiry

54445-64-6
20 suppliers
inquiry
![Cyclobutanecarboxaldehyde, 2-[(acetyloxy)methyl]-, (1R,2R)- (9CI)](/CAS/GIF/371784-53-1.gif)
371784-53-1
8 suppliers
inquiry