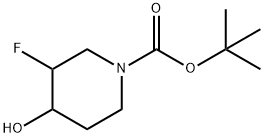
1-PIPERIDINECARBOXYLIC ACID, 3-FLUORO-4-HYDROXY-, 1,1-DIMETHYLETHYL ESTER synthesis
- Product Name:1-PIPERIDINECARBOXYLIC ACID, 3-FLUORO-4-HYDROXY-, 1,1-DIMETHYLETHYL ESTER
- CAS Number:373604-28-5
- Molecular formula:C10H18FNO3
- Molecular Weight:219.25
211108-50-8

373604-28-5
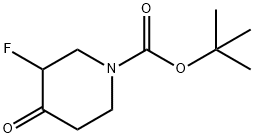
General procedure for the synthesis of tert-butyl 3-fluoro-4-hydroxypiperidine-1-carboxylate from tert-butyl 3-fluoro-4-oxopiperidine-1-carboxylate: To a solution of tert-butyl 3-fluoro-4-oxopiperidine-1-carboxylate (2.0 g, 9.2 mmol, 1.0 equiv.) in methanol (15 mL) was slowly added sodium borohydride (525 mg, 13.8 mmol, 1.5 equiv.) at 0 °C. 1.5 equiv). The reaction mixture was stirred at room temperature for 4 hours. After the reaction was completed, the reaction mixture was diluted with water (80 mL) and extracted with ethyl acetate (100 mL x 2). The organic layers were combined, washed with brine, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The crude product obtained was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 5:1) to give tert-butyl 3-fluoro-4-hydroxypiperidine-1-carboxylate (1.8 g, 90% yield) as a yellow solid.ESI-MS (M + H-56): 164.1.

211108-50-8
191 suppliers
$8.00/250mg

373604-28-5
89 suppliers
$55.00/250mg
Yield: 90%
Reaction Conditions:
with sodium tetrahydroborate in methanol at 0 - 20; for 4 h;
Steps:
213.1 1. Synthesis of tert-butyl 3-fluoro-4-hydroxypiperidine-1-carboxylate
[0600j To the solution of tert-butyl 3-fluoro-4-oxopiperidine-1-carboxylate (2.0 g, 9.2 mmol, 1.0 equiv) in MeOH (15 mL), NaBH4(525 mg, 13.8 mmol, 1.5 equiv) was slowly added at 0 °C. The reaction mixture was stirred at rt for 4 h. After diluting with water (80 mL), the mixture was extracted with ethyl acetate (100 mL x 2). The combined organic layers were washed with brine, dried, concentrated and purified by silica gel column chromatography (petroleum ether: EtOAc = 5:1) to give tert-butyl 3 -fluoro-4-hydroxypiperidine- 1 -carboxylate (1.8 g, yield: 90%) as a yellow solid. ESI-MS (M+H-56): 164.1.
References:
BIOGEN IDEC MA INC.;SUNESIS PHARMACEUTICALS, INC.;HOPKINS, Brian, T.;MA, Bin;CHAN, Timothy, Raymond;SUN, Lihong;ZHANG, Lei;KUMARAVEL, Gnanasambandam;LYSSIKATOS, Joseph, P.;KOCH, Kevin;MIAO, Hua WO2015/89337, 2015, A1 Location in patent:Paragraph 0600
![tert-Butyl 4-[(Trimethylsilanyl)oxy]-3,6-dihydro-2H-pyridine-1-carboxylate](/CAS/GIF/211108-48-4.gif)
211108-48-4
36 suppliers
$200.00/1g

373604-28-5
89 suppliers
$55.00/250mg

79099-07-3
0 suppliers
$5.00/5g

373604-28-5
89 suppliers
$55.00/250mg