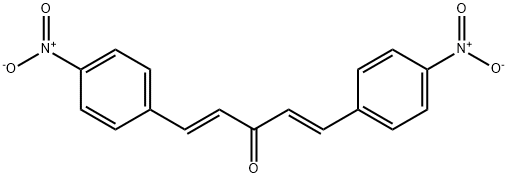
1,4-Pentadien-3-one, 1,5-bis(4-nitrophenyl)-, (E,E)- synthesis
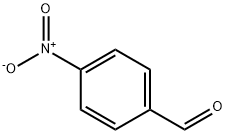
- Product Name:1,4-Pentadien-3-one, 1,5-bis(4-nitrophenyl)-, (E,E)-
- CAS Number:37630-48-1
- Molecular formula:C17H12N2O5
- Molecular Weight:324.29
Yield:37630-48-1 74.3%
Reaction Conditions:
with sodium hydroxide in ethanol;water; for 2 h;
Steps:
4.2.1. General Procedure for the Synthesis Curcumin Analogs
General procedure: Synthesis of mono-carbonyl curcumin analogs was accomplished using substituted aldehydes (4-methylbenzaldehyde 0.235 mL, 4-methoxybenzaldehyde 0.243 mL, 4-chlorobenzaldehyde0.281 gm, 4-(Dimethylamino)benzaldehyde) 0.298 gm, and 4-nitrobenzaldehyde0.302 gm) with 0.074 mL of acetone. A mixture of 2 mmol aldehydes was reacted with 1 mmol of the respective ketone in a 2:1 ratio using 15 mL cold ethanol as solvent and adding 40% of 10 mL sodium hydroxide aqueous solution and continuously stirring for 2 h. TLC plates were used for the reaction process. Finally, HCl (50%) solution 10 mL was added to neutralize the catalyst. The filtered dried product was recrystallized in ethyl acetate or ethanol [36].4.2.2. Synthesis of (1E,4E)-1,5-di-p-tolylpenta-1,4-dien-3-one (h1) Yield: 67.8%, yellow needle crystals, melting point: 173-176 °C, solubility: chloroform,ethyl acetate, Rf value: 0.80 in ethyl acetate n-hexane (3:7) mixture.
References:
Abdulaziz, Osama;Ahmad, Shujaat;Alghamdi, Saad;Almehmadi, Mazen;Ghias, Mehreen;Hussain, Haya;Kamal, Zul;Khan, Farman Ali;Khan, Nasir Mehmood;Muhammad, Juma;Rahman, Shafiq Ur;Shah, Syed Wadood Ali;Ullah, Abid [Molecules,2021,vol. 26,# 23,art. no. 7168]