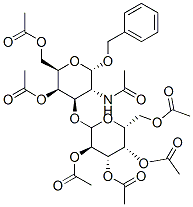
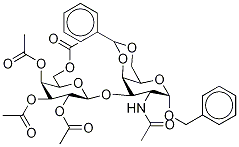
Benzyl 2-Acetamido-4,6-di-O-acetyl-3-O-(2,3,4,6-tetra-O-acetyl--D-galactosyl)-2-deoxy-a-D-galactopyranoside synthesis
- Product Name:Benzyl 2-Acetamido-4,6-di-O-acetyl-3-O-(2,3,4,6-tetra-O-acetyl--D-galactosyl)-2-deoxy-a-D-galactopyranoside
- CAS Number:3809-10-7
- Molecular formula:C33H43NO17
- Molecular Weight:725.69

108-24-7
0 suppliers
$14.00/250ML
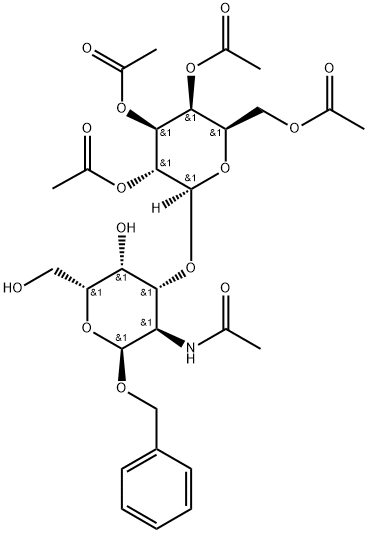
90754-57-7
19 suppliers
$89.29/5mg

3809-10-7
14 suppliers
$95.00/10mg
Yield:3809-10-7 98%
Reaction Conditions:
with pyridine at 20;
Steps:
13 3.13 Benzyl 2-acetamido-4,6-di-O-acetyl-2-deoxy-3-O-(2,3,4,6-tetra-O-acetyl-β-D-galactopyranosyl)-α-D-galactopyranoside (14)
A solution of 12 (200mg, 0.31mmol) in a mixture of dry pyridine (1mL) and Ac2O (1mL) was stirred overnight at rt, ethanol (10mL) and toluene (10mL) was added and the reaction mixture was evaporated to dryness. The syrupy residue was subjected to column chromatography (toluene→acetone) affording 14 as a foam (220mg, 98%): Rf 0.5 (toluene/EtOAc 2:1); [α]D20+85.3; HRESIMS: m/z=726.2613 [M+H]+, calcd for C33H43NO17: 725.2531; m/z=748.2425 [M+Na]+, calcd for C33H43NO17Na: 748.2429; 1H NMR (Gal=II, GalNAc=I): δ 7.40-7.30 (m, 5H, H2h); 5.64 (d, 1H, J 9.0Hz, NH); 5.38 (bd, 1H, J3,4 3.0Hz, H-4I); 5.34 (bd, 1H, J3,4 3,6Hz, H-4II); 5.12 (dd, 1H, J2,1 8.40Hz, J2,3 10.2Hz, H-2II); 5.04 (d, 1H, J1,2 4.2Hz, H-1I); 4.95 (dd, 1H, J3,4 3.6Hz, J2,3 10.2Hz, H-3II); 4.58 (d, 1H, J1,2 8.4Hz, H-1II); 4.55-4.51 (m, 1H, H-2I); 4.20-4.10 (m, 3H, H-5I, H-6′I, H-6II); 4.04-4.01 (m, 1H, H-6I); 4.00 (dd, 1H, J3,4 3.0Hz, J3,2 11.4Hz, H-3I); 3.86-3.85 (m, 1H, H-5II); 2.14-1.96 (m, 21H, COCH3, NHCOCH3); 13 NMR: δ 100.5 (C-1II), 97.2 (C-1I), 72.8 (C-3I), 71.0 (C-5I), 70.9 (C-3II), 70.8 (CH2Ph), 68.8 (C-4I), 68.5 (C-2II), 67.6 (C-5I), 66.8 (C-4II), 62.9 (C-6I), 61.1 (C-6II), 48.5 (C-2I), 23.3 (NHCOCH3), 20.5 (COCH3).
References:
Torgov, Vladimir;Danilov, Leonid;Utkina, Natalia;Veselovsky, Vladimir;Brockhausen, Inka [Carbohydrate Research,2017,vol. 453-454,p. 19 - 25]

100-51-6
1434 suppliers
$5.00/100g

3809-10-7
14 suppliers
$95.00/10mg
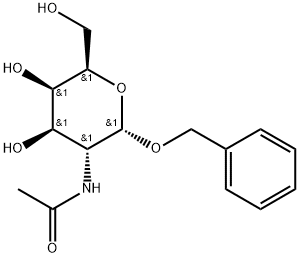
3554-93-6
78 suppliers
$70.00/25mg

3809-10-7
14 suppliers
$95.00/10mg
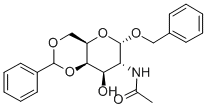
3554-91-4
23 suppliers
$175.00/25mg

3809-10-7
14 suppliers
$95.00/10mg
86327-84-6
15 suppliers
$125.09/10mg

3809-10-7
14 suppliers
$95.00/10mg