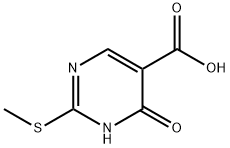
4-Hydroxy-2-(methylthio)pyrimidine-5-carboxylic acid synthesis
- Product Name:4-Hydroxy-2-(methylthio)pyrimidine-5-carboxylic acid
- CAS Number:397308-78-0
- Molecular formula:C6H6N2O3S
- Molecular Weight:186.19
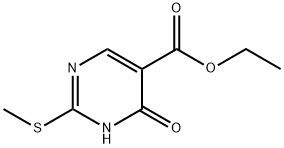
5909-24-0
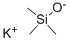
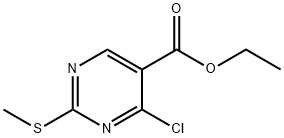
10519-96-7

397308-78-0
A. Over 20 min, 4-chloro-2-methylthiopyrimidine-5-carbonyl chloride was slowly added to a suspension of potassium trimethylmethylsilyl oxide (90% purity, 40 g, 0.31 mol) in 1,2-dimethoxyethane (300 mL), along with ethyl 4-chloro-2-methylthio-5-pyrimidine carboxylate (Aldrich, 15 g, 64 mmol) in a 1 ,2-dimethoxyethane (100 mL) solution. This addition process is accompanied by a mild exotherm and requires the use of an ice bath to maintain the reaction temperature at ambient temperature. After addition, the reaction mixture was stirred at ambient temperature for 1 hour, followed by heating and refluxing for 36 hours, after which it was cooled to ambient temperature. The reaction mixture was quenched with 1 M HCl (aq) and subsequently extracted with ethyl acetate and the organic phase was dried over sodium sulfate to give the crude product (11 g). The crude product was recrystallized by ethyl acetate to give 4-hydroxy-2-methylthiopyrimidine-5-carboxylic acid as a white solid (8.8 g, 47 mmol, 73% yield). m/z MS (-ESI) 185 (M-, 100). A portion of the product (3.00 g, 16.1 mmol) was taken and mixed with thionyl chloride (90 mL), catalyzed by the addition of DMF (0.20 mL) and the mixture was heated to reflux. After refluxing for 1 hour, it was cooled to ambient temperature and concentrated in vacuum to give a beige solid. The solid was sequentially ground with hot toluene and hot hexane (i.e., the soluble portion of both solvents was the target product), and vacuum concentration afforded 4-hydroxy-2-(methylthio)pyrimidine-5-carboxylic acid as a white solid (3.90 g, 15.6 mmol, 97% yield).1H NMR (300 MHz, CDCl3) δ 9.15 (s, 1H), 2.63 (s, 3H).
53554-29-3
202 suppliers
$6.00/1g

397308-78-0
93 suppliers
$29.00/250mg
Yield:397308-78-0 82.6%
Reaction Conditions:
with sodium hydroxide at 80;Temperature;
Steps:
4-6 Example 6:
Put 38.6g (18mmol) of ethyl 4-hydroxy-2-methylthiopyrimidine-5-carboxylate into a round-bottomed flask, add 238ml of sodium hydroxide solution (0.3mol/L) dropwise, and reflux at 80°C After the reaction was completed, the reaction solution was transferred to a beaker, the pH was adjusted to neutrality with dilute hydrochloric acid, the reaction solution was concentrated under reduced pressure, filtered under reduced pressure, and the filter cake was washed three times with ice water, and the filter cake was dried to no After weight reduction, 26.9 g of a white solid was obtained with a yield of 82.6%.
References:
CN114933567,2022,A Location in patent:Paragraph 0049-0052; 0060

5909-24-0
417 suppliers
$6.00/5g

397308-78-0
93 suppliers
$29.00/250mg

5909-24-0
417 suppliers
$6.00/5g
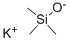
10519-96-7
275 suppliers
$6.00/1g

397308-78-0
93 suppliers
$29.00/250mg

53554-29-3
202 suppliers
$6.00/1g

397308-78-0
93 suppliers
$29.00/250mg