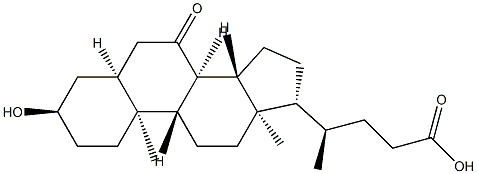
3alpha-Hydroxy-7-oxo-5beta-cholanic Acid synthesis
- Product Name:3alpha-Hydroxy-7-oxo-5beta-cholanic Acid
- CAS Number:4651-67-6
- Molecular formula:C24H38O4
- Molecular Weight:390.56
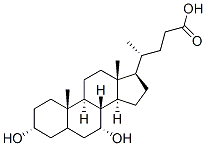
474-25-9

4651-67-6
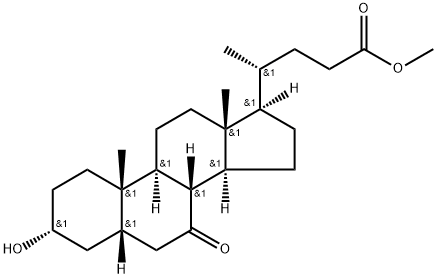
The general procedure for the synthesis of (R)-4-((3R,5S,8R,9S,10S,13R,14S,17R)-3-hydroxy-10,13-dimethyl-7-oxohexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoic acid from goose deoxycholic acid was as follows: goose deoxycholic acid (5 g, 12.7 mmol) was placed in a reaction flask and dichloromethane ( 50 mL) as solvent, followed by DMSO (1.12 g, 14.3 mmol) and triethylamine (4.2 g, 41.5 mmol) sequentially. After cooling the reaction system to -30 °C, trifluoroacetic anhydride (3.2 g, 15.2 mmol) was added slowly and dropwise. The reaction mixture was stirred at -30 °C for 1.5 hours. Upon completion of the reaction, the reaction was quenched by the addition of water (10 mL) and the mixture was gradually warmed to room temperature and stirred. Extraction was carried out with methanol (20 mL x 2), the organic phases were combined, washed once with saturated sodium chloride solution, dried over anhydrous sodium sulfate, filtered and the organic phase was concentrated to dryness to give the target product (R)-4-((3R,5S,8R,9S,10S,13R,14S,17R)-3-hydroxy-10,13-dimethyl-7-oxohexadecahydro-1H-cyclopenta[a]phenanthren- 17-yl)pentanoic acid (Compound 2) in a yield of 4.89 g in 98.6%.
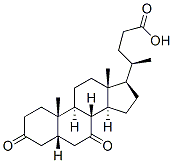
859-97-2
32 suppliers
inquiry

4651-67-6
349 suppliers
$39.00/500mg
Yield:4651-67-6 98.8%
Reaction Conditions:
with D-Glucose;sodium hydroxide in propane-1,2,3-triol at 30 - 35; pH=7 - 8; for 2 h;Enzymatic reaction;Solvent;
Steps:
1-3 Example 2
Add 300ml of water to a clean reaction flask, add 50.0g of compound I (purity: 99.1%) and 50.0ml of glycerol with stirring,Adjust pH=78 with 2N sodium hydroxide, after compound I is completely dissolved, adjust the reaction temperature to 3035 , add 120g glucose,10g 3α reductase, 22g glucose dehydrogenase, 1.5g coenzyme one, 1.5g coenzyme two.After stirring uniformly, adjust pH=7-8 with 2N sodium hydroxide solution, and react for 2 hours, until the content of compound I monitored by HPLC is 0.1%.After the reaction, the temperature of the system was raised to 70-80° C., and the temperature was kept stirring for 30-60 min.The filtrate was collected by suction filtration while hot, and the filter cake was again heated to 70-80° C. with 100 ml of water, kept stirring for 30-60 min, and the filtrate was combined after hot filtration.The filtrate was concentrated under reduced pressure at 50-60°C to remove glycerol, cooled to 0-10°C, and 2N hydrochloric acid was slowly added dropwise to adjust pH=2-3, a large amount of solid was precipitated, and the temperature was kept stirring for 30min-60min.Filter, rinse with a small amount of tap water, and dry the filter cake in a hot air circulating oven at 50°C until it is qualified.Dry weight: 49.4 g, LC purity: 98.1%.
References:
CN113968891,2022,A Location in patent:Paragraph 0024-0032

474-25-9
718 suppliers
$20.80/5G

4651-67-6
349 suppliers
$39.00/500mg
10538-59-7
135 suppliers
inquiry

4651-67-6
349 suppliers
$39.00/500mg
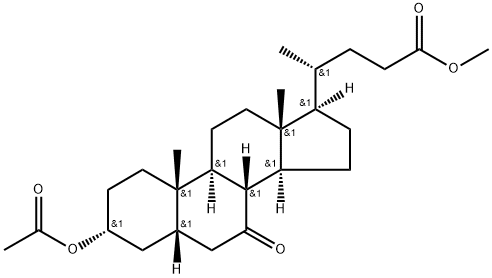
10452-65-0
7 suppliers
inquiry

4651-67-6
349 suppliers
$39.00/500mg