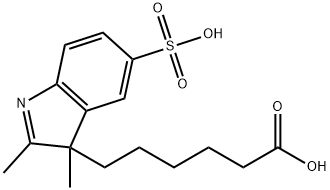
3H-Indole-3-hexanoic acid, 2,3-dimethyl-5-sulfo- synthesis
- Product Name:3H-Indole-3-hexanoic acid, 2,3-dimethyl-5-sulfo-
- CAS Number:407627-51-4
- Molecular formula:C16H21NO5S
- Molecular Weight:339.41
Yield:407627-51-4 40%
Reaction Conditions:
with glacial acetic acid for 6 - 12 h;Heating / reflux;
Steps:
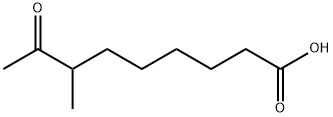
5; 13.1 13.1 6-(2,3-dimethyl-5-sulfo-3H-indol-3-yl)hexanoic acid
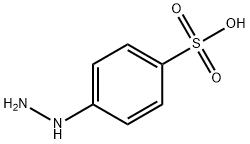
To a stirred solution of 4-hydrazinobenzenesulfonic acid (ALDRICH, LU,,. 25 g, 0.06 mol) in acetic acid (50 ml) was added 7-ACETYLOCTANOIC acid (16.7 g, 0.09 MOL). The reaction mixture was heated under reflux for 12 hrs. Acetic acid was removed under reduced pressure. The resulting solid was dissolved in methanol and reprecipitated with a saturated solution of potassium hydroxide in isopropanol. The solid was filtered, washed with isopropanol and dried, (8 g, 40%). The analytical sample was obtained by C18 reversed phase column chromatography using water/methanol mixture as solvent., m. p. 250 °C dec; IR v cm-1 = 2930,2597, and 1719. H NMR, D20, 6, 7.8-7. 9 (m, 2H, 4-H and 6-H of aromatic protons); 7.6 (d, J = 7Hz, 1H, 7-H of aromatic); 2.2 (T, J =7Hz, 2H,-CH2-COOH) ; 1.9-2. 1 (m, 2H, alkyl) ; 1.2-1. 6 (a singlet merged in a multiplet, 7H,-CH3 & (-CH2) 2) ; 0.6-0. 9 (m, 2H, alkyl.); 4-Hydrazinobenzenesulfonic acid (1.88g, 10mmol), 7-methyl-8-oxononanoic acid (2.8g, 15MMOL) and glacial acetic acid (10ml) were mixed and heated under reflux for 6hrs. The solvent was then evaporated under vacuum and the residue triturated with diethyl ether until a solid was obtained. This was dried under vacuum to give crude product, 3. 4G (100%). This was purified by preparative HPLC as required (RPC18. Water+0. 1%TFANo.MECN+0.1% TFA gradient). UVNis (Water+0.1% TFA): 274,229, 204NM. 1H-NMR (D20) 6 0.6-0. 9 (2H, broad M), 1.10- 1.25 (2H, broad m), 1.35-1. 50 (2H, m), 1.60 (3H, s), 2.10-2. 40 (2H, broad m + 2H, t), 7.77 (1 H, d), 7.97 (1 H, dd) and 8.06 (1 H, d) MS (MALDI-TOF) MH+ 340.
References:
WO2004/39894,2004,A2 Location in patent:Page 24; 31
25542-62-5
257 suppliers
$6.00/5g

407627-51-4
32 suppliers
$309.00/1g
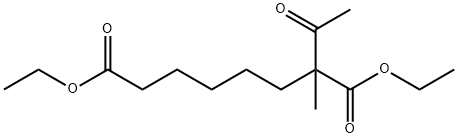
407627-95-6
1 suppliers
inquiry

407627-51-4
32 suppliers
$309.00/1g