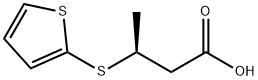
(3S)-3-(2-thienylthio)butanoic acid synthesis
- Product Name:(3S)-3-(2-thienylthio)butanoic acid
- CAS Number:133359-80-5
- Molecular formula:C8H10O2S2
- Molecular Weight:202.29
1197375-23-7
12 suppliers
inquiry

133359-80-5
57 suppliers
inquiry
Yield: 99 % ee
Reaction Conditions:
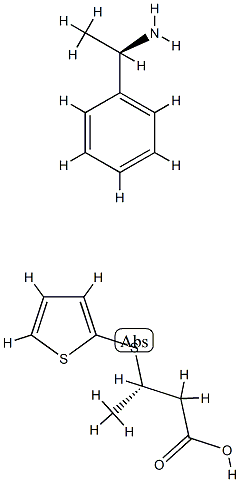
Stage #1:D-(+)-methylbenzylamine (3S)-3-(2-thienylthio)butyrate salt with sodium hydroxide in water;toluene; pH=11 - 12
Stage #2: with hydrogenchloride in water;toluene; pH=1 - 2Product distribution / selectivity;
Steps:
2 Obtaining (3S)-3-(2-thienylthio) butyric acid
22.07 g (0.07 moles) of the resolved D-(+)-methylbenzylamine (3S)-3-(2-thienylthio)butyrate salt were added to 84 mL of toluene and 84 mL of water, the pH of the dissolution was adjusted to 11-12 with an aqueous solution of NaOH, the mixture was kept under stirring until achieving complete dissolution and the obtained phases were separated. The aqueous phase was mixed with 84 mL of toluene and kept under stirring, the pH was adjusted to 1-2 with an aqueous solution of concentrated HCl, and the phases were separated. The organic phase was concentrated to a residue, 13.17 g of the compound of the title in the form of oil being obtained. The enantiomeric excess is measured by means of the transformation of (3S)-3-(2-thienylthio) butyric acid into a diastereoisomeric salt mixture by HPLC (High Performance Liquid Chromatography); these salts are obtained by means of dissolving the obtained oil in toluene by adding D-(+)-methylbenzylamine and EEDQ (N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline), heating to reflux, cooling, distilling the toluene and dissolving in acetonitrile. The enantiomeric excess is 99%. 1H NMR (CDCl3): δ 11.08 (1H, bs), 7.40 (1H, dm), 7.17 (1H, dm), 7.03 (1H, m), 3.35 (1H, m), 2.65 (1H, dd), 2.41 (1H, dd), 1.32 (3H, d)
References:
Ragactives, S.L. EP2128161, 2009, A1 Location in patent:Page/Page column 10
133359-79-2
9 suppliers
inquiry

133359-80-5
57 suppliers
inquiry