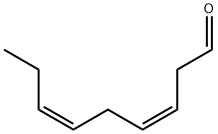
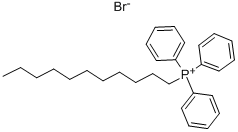
(3Z,6Z,9Z)-3,6,9-Icosatriene synthesis
- Product Name:(3Z,6Z,9Z)-3,6,9-Icosatriene
- CAS Number:87255-14-9
- Molecular formula:C20H36
- Molecular Weight:0
Yield:87255-14-9 62%
Reaction Conditions:
with sodium hexamethyldisilazane in tetrahydrofuran at -78 - 20;Inert atmosphere;Wittig Olefination;
Steps:
(3Z,6Z,9Z)-Eicosa-3,6,9-triene 14 (Bestmann et al., 1987;Chattopadhyay and Mamdapur, 1990; Wang and Zhang,2007; Heath et al., 1983) (0.25 g, 62%) was prepared asabove from triphenyl(undecyl)phosphonium bromide (0.43 g,0.86 mmol), (3Z,6Z)-nona-3,6-dienal 9 (0.1 g, 0.7 mmol) and sodium bis(trimethylsilyl)amide (1.3 mL, 1.3 mmol) in dry THF(20 mL)at -78°C under nitrogen. Themixture was allowed to reach room temperature and stirred for 30 min, then cooled again to -78°C and (3Z,6Z)-nona-3,6-dienal9 (0.15 g, 1 mmol) in dry THF (3 mL) was added. The mixture wasstirred and allowed slowly to reach room temperature, then cooledto 0°C and quenched with sat.aq. NH4Cl (15 mL). The product was extracted with ethyl acetate (3 × 50 mL). The combined organic layers were dried and evaporated. The residue was purified by column chromatography on silica eluting with petrol to give the title compound 14. It showed 1H(400 MHz, CDCl3): 5.43-5.29 (6H, m), 2.82(4H, t, J 6 Hz), 2.07 (4H, m), 1.27 (16H, m), 0.98 (3H, t, J 7.5 Hz), 0.88(3H, t, J 6.5 Hz); δc (101 MHz, CDCl3): 131.9, 130.4, 128.3, 128.2,127.6, 127.1, 31.9, 29.64, 29.63, 29.5, 29.34, 29.32, 27.2, 25.6, 25.5,22.6, 20.5, 14.2, 14.1; vmax: 3012, 2925, 2854, 1647, 1493, 1399,875 cm-1; M+: 276.
References:
Mustafa, Hussein H.;Baird, Mark S.;Al Dulayymi, Juma'A R.;Tverezovskiy, Viacheslav V. [Chemistry and Physics of Lipids,2014,vol. 183,p. 34 - 42]

1638152-99-4
0 suppliers
inquiry

87255-14-9
0 suppliers
inquiry
696-86-6
2 suppliers
inquiry

87255-14-9
0 suppliers
inquiry

1638153-00-0
0 suppliers
inquiry

87255-14-9
0 suppliers
inquiry