
4-[2-(2-OXO-PYRROLIDIN-1-YL)-ETHOXY]-BENZOIC ACID synthesis
- Product Name:4-[2-(2-OXO-PYRROLIDIN-1-YL)-ETHOXY]-BENZOIC ACID
- CAS Number:215656-70-5
- Molecular formula:C13H15NO4
- Molecular Weight:249.26
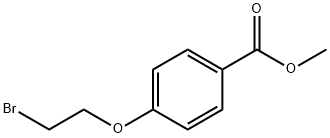
56850-91-0
36 suppliers
$60.00/100mg
![4-[2-(2-OXO-PYRROLIDIN-1-YL)-ETHOXY]-BENZOIC ACID](/StructureFile/ChemBookStructure8/GIF/CB2725659.gif)
215656-70-5
7 suppliers
$315.00/500mg
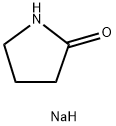
3195-95-7
0 suppliers
inquiry
Yield:215656-70-5 46%
Reaction Conditions:
tetra-(n-butyl)ammonium iodide in tetrahydrofuran;
Steps:
7.A A.
After 1 h, the suspension of the sodium salt of 2-pyrrolidinone thus formed was added to a solution of methyl 4-(2-bromoethoxy)benzoate (1.17 g, 4.52 mmol) and a catalytic amount of tetrabutylammonium iodide in dry THF (10 mL). The reaction was heated at reflux for 18 h. The reaction mixture was concentrated under reduced pressure then the residue partitioned between ethyl acetate (150 mL) and 2 N sodium hydroxide (50 mL). The aqueous layer was made acidic to pH 3 with conc. HCl; then the solvent was removed under reduced pressure. The resulting solid was triturated with ethyl acetate to give the acid as a white solid (523 mg, 46%). 1H-NMR (300 MHz, DMSO-d6 δ7.90 (d, J=9.0 Hz, 2H), 7.01 (d, J=9.0 Hz, 2H), 4.18 (t, J=6.0 Hz, 2H), 3.58 (t, J=6.0 Hz, 2H), 3.41(t, J=8.0 Hz, 2H), 3.26 (bs, 1H), 2.21 (t, J=8.0 Hz, 2H) 4.18 (q, J=8.0 Hz, 2H).
References:
US6541499,2003,B1