
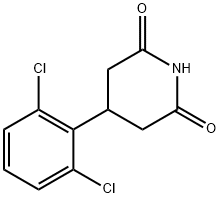
4-(2,6-dichloro-phenyl)-piperidine hydrochloride synthesis
- Product Name:4-(2,6-dichloro-phenyl)-piperidine hydrochloride
- CAS Number:371981-23-6
- Molecular formula:C11H14Cl3N
- Molecular Weight:266.59
371981-22-5
4 suppliers
inquiry

371981-23-6
16 suppliers
$351.00/1g
Yield:371981-23-6 87%
Reaction Conditions:
Stage #1: 4-(2,6-dichlorophenyl)piperidine-2,6-dionewith dimethylsulfide borane complex in tetrahydrofuran; for 3 h;Inert atmosphere;Reflux;
Stage #2: with hydrogenchloride in tetrahydrofuran; for 3 h;Reflux;
Steps:
4-(2,6-Dichlorophenyl)piperidine Hydrochloride (3·HCl)
To a solution of imide 13 (1.12 g, 4.34 mmol) in anhyd THF (50 mL)cooled in ice under N2 was added dropwise 2 M BH3·SMe2 in THF (21.7mL, 43.4 mmol). Upon completion of the addition, the mixture waswarmed to r.t. then heated at reflux for 3 h. The mixture was thencooled in ice and carefully quenched with 2 N HCl, then heated at refluxagain for 3 h. The mixture was cooled and the volatile solventswere removed under reduced pressure. The mixture was diluted withwater, the pH adjusted to >7 with 2 N NaOH solution then extractedwith EtOAc (3 ×). The combined extracts were dried (MgSO4) and thesolvent was removed under reduced pressure. The crude was takenup in CH2Cl2 and 2 N HCl in Et2O was added until acidic, then all thesolvents were removed under reduced pressure. The residue was triturated(Et2O) and the solid formed was collected by filtration as thepiperidine 3 (1.01 g, 87%). 1H and 13C NMR data match that in the literature.211H NMR (300 MHz, CDCl3): δ = 9.53-10.01 (m, 2 H), 7.22-7.37 (m, 2H), 7.11 (t, J = 8 Hz, 1 H), 3.71-3.84 (m, 1 H), 3.57-3.71 (m, 2 H), 2.91-3.19 (m, 4 H), 1.82 (m, 2 H).13C NMR (75 MHz, CDCl3): δ = 137.0, 135.3, 130.3, 128.6, 44.8, 38.3,24.8.
References:
Perrey, David A.;Li, Jun-Xu;Zhang, Yanan [Synthesis,2017,vol. 49,# 6,art. no. SS-2016-M0600-OP,p. 1394 - 1400]