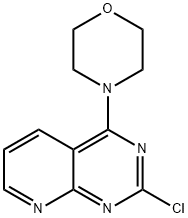
4-(2-CHLOROPYRIDO[2,3-D]PYRIMIDIN-4-YL)MORPHOLINE synthesis
- Product Name:4-(2-CHLOROPYRIDO[2,3-D]PYRIMIDIN-4-YL)MORPHOLINE
- CAS Number:908142-01-8
- Molecular formula:C11H11ClN4O
- Molecular Weight:250.68
Yield:908142-01-8 100%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 20; for 6 h;
Steps:
1
3-(2-Chloroquinazolin-4-yl)-8-oxa-3-azabicyclo[3.2.1]octane (2) (1.0 g), 4- aminophenylboronic acid, pinacol ester (660 mg), toluene (7.5 mL), EtOH (7.5 mL), 2M aqueous Na2COs and tetrakis(thphenylphosphine) palladium (230 mg) were heated by microwave irradiation (1 hour, 120° C). After cooling, the reaction mixture was partitioned between ethyl acetate and water. The organic extracts were washed with brine and dried over MgSO4 and concentrated in vacuo to result in a dark syrup. Purification by RP HPLC gave the title compound as a yellow foam (0.52 g). A mixture of 2,4-dichloro-pyhdo[2,3-d]pyrimidine (200 mg, 1.00 mmol), morpholine (104mg, 1.20mmol) and triethyl amine (120 mg, 1.20 mmol) in 4 mL of THF was stirred at room temperature for 6 hours. Then the reaction mixture was diluted with 200 mL of ethyl acetate and the organic layer was washed with saturated sodium bicarbonate aqueous solution and brine. The organic layer then was collected and dried over anhydrous sodium sulfate and concentrated to give 2-chloro-4-morpholin-4-yl-pyrido [2, 3-d] pyrimidine (4, 250mg, 100% yield) as a light yellow solid. HPLC: RT = 0.27 min; MS 251 , 253 [M+H].
References:
WO2010/120987,2010,A1 Location in patent:Page/Page column 40

![2,4-DICHLOROPYRIDO[2,3-D]PYRIMIDINE](/CAS/GIF/126728-20-9.gif)