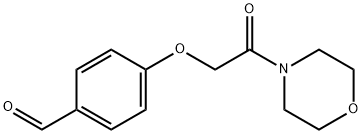
4-(2-MORPHOLIN-4-YL-2-OXO-ETHOXY)-BENZALDEHYDE synthesis
- Product Name:4-(2-MORPHOLIN-4-YL-2-OXO-ETHOXY)-BENZALDEHYDE
- CAS Number:30817-36-8
- Molecular formula:C13H15NO4
- Molecular Weight:249.26
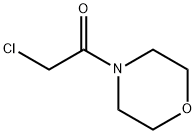
1440-61-5
170 suppliers
$10.00/1g
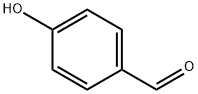
123-08-0
961 suppliers
$5.00/10g

30817-36-8
15 suppliers
$188.00/500mg
Yield:30817-36-8 100%
Reaction Conditions:
with potassium carbonate in tetrahydrofuran at 100; for 24 h;Heating / reflux;
Steps:
37.2 Step 2
Step 2 A THF (10 mL) solution of 4-hydroxybenzaldehyde (0.50 g, 4.09 mmol) was added with N-chloroacetylmorpholine (1.00 g, 6.12 mmol) obtained in Step 1 and potassium carbonate (1.70 g, 12.3 mmol), followed by heating under reflux at 100°C for 24 hours. The reaction mixture was concentrated, and the residue was added with ethyl acetate and saturated brine, and then was extracted. The organic layer was dried over anhydrous sodium sulfate, and was concentrated to obtain N-(4-formylphenoxy)acetylmorpholine (1.24 g, quantitative yield). 1H-NMR (270 MHz, CDCl3) δ 3.51-3.74 (m, 8H), 4.80 (s, 2H), 7.07 (d, J = 8.9 Hz, 2H), 7.85 (d, J = 8.9 Hz, 2H), 9.90 (s, 1H).
References:
EP1650194,2006,A1 Location in patent:Page/Page column 34-35