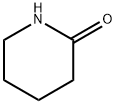
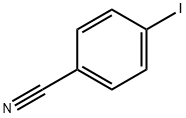
4-(2-OXO-PIPERIDIN-1-YL)-BENZONITRILE synthesis
- Product Name:4-(2-OXO-PIPERIDIN-1-YL)-BENZONITRILE
- CAS Number:186651-05-8
- Molecular formula:C12H12N2O
- Molecular Weight:200.2365
Yield:186651-05-8 93%
Reaction Conditions:
with caesium carbonate;palladium diacetate;4,5-bis(diphenylphosphino)-9,9-dimethylxanthene in 1,4-dioxane at 80; for 5 h;
Steps:
9.A Part A.
2-Piperidone (9.00 g, 90.79 mmol), 4-IODOBENZONITRILE (24.95 g, 108. 95 mmol), cesium carbonate (44.37 g, 136. 18 mmol), palladium (II) acetate (4. 08 g, 18. 16 NUNOL), AND 9, 9-DIMETHYL-4, 5-bis (DIPHENYLPHOSPHINO) XANTHENE (15.76 g, 27. 24 MMOL) WERE CHARGED TO a round bottom flask and flushed with N2. Degassed 1, 4-dioxan (100 mL) was added to the flask, and the flask was flushed again with N2. The reaction was heated to 80°C for 5HR. The reaction was cooled to rt and concentrated to dryness. The crude material was dissolved in water (250 ML) and ethyl acetate (250 mL), extracted with ethyl acetate (3X200 mL), washed with brine (1X250 mL), dried with MGSO4, and concentrated. Purification by flash chromatography using 0-100% ethyl ACETATE/HEXANES gradient as the eluent afforded 16.84 g (93%): IHNMR (CDCS 7.67 (D, J=8. 8HZ, 2H), 7.43 (D, J=8. 8HZ, 2H), 2.59 (T, J=6. 2Hz, 2H), 1.98-1. 92 (m, 4H) ppm.
References:
WO2004/83174,2004,A2 Location in patent:Page 148
1575-61-7
380 suppliers
$14.00/1g

186651-05-8
8 suppliers
inquiry
873-74-5
654 suppliers
$9.00/1g

186651-05-8
8 suppliers
inquiry