
4-(3-Cyclohexen-1-yl)-3-buten-2-one synthesis
- Product Name:4-(3-Cyclohexen-1-yl)-3-buten-2-one
- CAS Number:2817-96-1
- Molecular formula:
- Molecular Weight:0
Yield:2817-96-1 72.9%
Reaction Conditions:
with sodium hydroxide in water at 70; for 4 h;
Steps:
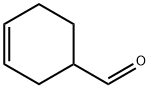
1 EXAMPLE 1 : Synthesis of 4-(cydohex-3-en-l-yI)but-3-en-2-one
Example 1 provides a synthesis of a compound of Formula Ila, wherein X=0, R2=methyl, R3=R5=H, and R6=cyclohexenyl. This compound is an intermediate for synthesis of 4-(cyclohex-3-en-l-yl)but-3-en-2-ol and 4-(cyclohex-3-en-l-yl)but-3-en-2-yl acetate as described in Example 2 and Example 3 below.Cyclohex-3-ene-l-carbaldehyde (70g, 1 eq.), acetone (95.2mL), 1% NaOH (aq.) (l54mL) and water (l26mL) were combined in a round bottom flask and heated at 70°C for 4 hours. The mixture was subsequently cooled to room temperature, and additional 100 mL of water was added. The reaction mixture was extracted with ethyl acetate, dried over MgS04, filtered and concentrated to obtain 97g of crude oil. The oil was distilled (0.07T, 5l°C) to afford 69.6g of 4-(cyclohex-3-en-l-yl)but-3-en-2-one (72.9% yield).
References:
WO2019/236614,2019,A1 Location in patent:Page/Page column 33; 34
100-50-5
206 suppliers
$27.00/5mL

2817-96-1
1 suppliers
inquiry
