
4-(3-Fluoro-2-nitrophenyl)morpholine synthesis
- Product Name:4-(3-Fluoro-2-nitrophenyl)morpholine
- CAS Number:179900-21-1
- Molecular formula:C10H11FN2O3
- Molecular Weight:226.2
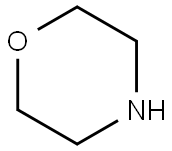
110-91-8
710 suppliers
$9.00/1g
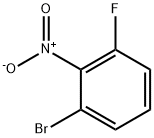
886762-70-5
236 suppliers
$11.00/1g

179900-21-1
9 suppliers
$292.00/1g
Yield:179900-21-1 42%
Reaction Conditions:
with palladium diacetate;Cs2CO3;4,5-bis(diphenylphosphino)-9,9-dimethylxanthene in 1,4-dioxane at 110; for 8 h;Buchwald-Hartwig Coupling;
Steps:
11.A Step A
Commercially available 2-bromo-6-fluoro-nitrobenzene (1.5 g, 6.82 mmol) was combined with palladium(ll) acetate (0.16 g, 0.8 mmol), 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene (0.62 g, 0.8 mmol) and cesium carbonate (4.44 g, 13.64 mmol). To the mixture was then added degassed 1 ,4-dioxane (15 mL) and morpholine (0.84 g, 9.6 mmol). The reaction mixture was heated at ~1 10°C in a sand-bath for 8 h. The reaction mixture was cooled to room temperature and diluted with ethyl acetate (150 mL), water, (20 mL) and brine (20 mL). The organic phase was separated, dried over Na2S04, filtered and the solvents were removed under reduced pressure. The residue was purified on HP-Sil SNAP cartridges using a Biotage Isolera One purification system employing an EtOAc/n-heptane gradient (5/95 -> 40/60) to afford the title compound as a yellow oil (0.65 g, 42 %). 1H-NMR (400 MHz, CDCI3): δ = 3.02-3.06 (m, 4H), 3.77-3.81 (m, 4H), 6.91 -6.97 (m, 2H), 7.36-7.44 (m, 1 H)
References:
WO2015/110263,2015,A1 Location in patent:Page/Page column 149

110-91-8
710 suppliers
$9.00/1g
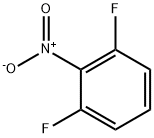
19064-24-5
278 suppliers
$6.00/1g

179900-21-1
9 suppliers
$292.00/1g