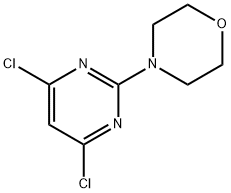
4-(4,6-Dichloropyrimidin-2-yl)morpholine synthesis
- Product Name:4-(4,6-Dichloropyrimidin-2-yl)morpholine
- CAS Number:10397-13-4
- Molecular formula:C8H9Cl2N3O
- Molecular Weight:234.08

110-91-8
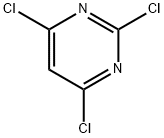
3764-01-0

10397-13-4
52127-83-0
2,4,6-Trichloropyrimidine (5) (3.158 g, 17.22 mmol) was mixed with acetone (60 mL) and the mixture was cooled to 0 °C. Morpholine (7) (1.05 equiv, 1.576 g, 18.09 mmol) was slowly added at 0 °C, followed by stirring of the reaction mixture at 0 °C for 15 min, then warmed up to room temperature and continued stirring for 15 min. The progress of the reaction was monitored by thin layer chromatography (TLC, the unfolding agent was a hexane solution of 20% ethyl acetate). After completion of the reaction, the reaction mixture was concentrated by rotary evaporator and further dried under high vacuum. The crude product was purified by silica gel column chromatography using hexane solution of 20% ethyl acetate as eluent to afford the major regional isomer 2-morpholino-4,6-dichloropyrimidine (10) (3.183 g, 13.6 mmol, 79% yield). Elemental analysis (C8H9N3OCl2) Calculated values: C, 41.05; H, 3.88; measured values: C, 42.27; H, 4.06. 1H-NMR (300 MHz, CDCl3) δ 6.40 (s, 1H), 3.82-3.70 (m, 4H), 3.65 (m, 4H). High Resolution Mass Spectrometry (HRMS) [M]+ Calculated value: C8H9N3OCl2, 234.0201; Measured value: 234.0196. secondary regioisomer 4-(2,6-dichloropyrimidin-4-yl)morpholine (11) (0.806 g, 3.444 mmol, 20% yield). 1H-NMR (300 MHz, CDCl3) δ 6.56 (s, 1H), 3.85-3.60 (m, 8H). 13C-NMR (101MHz, CDCl3) δ 161.75, 160.55, 108.31, 66.59, 44.39. HRMS [M]+ calculated value: C8H9N3OCl2, 234.0201; measured value: 234.0196.

110-91-8
710 suppliers
$9.00/1g

3764-01-0
398 suppliers
$6.00/5g

10397-13-4
163 suppliers
$11.00/250mg

52127-83-0
73 suppliers
$95.00/1 g
Yield: 79% , 20%
Reaction Conditions:
in acetone at 0 - 20; for 0.5 h;
Steps:
3.1.4. 4-(2,6-Dichloropyrimidin-4-yl)morpholine, (10) and 4-(4,6-dichloropyrimidin-2-yl)morpholine, (11)
2,4,6-Trichloropyrimidine (5) (3.158 g, 17.22 mmol) and acetone (60 mL) were combined and cooledto 0 °C. Morpholine (7) (1.05 eq., 1.576 g, 18.09 mmol) was added and the solution stirred at 0 °C for15 min then warmed to room temperature for another 15 min. The reaction was monitored by TLCanalysis (20% ethyl acetate in hexanes). The reaction was then concentrated via rotary evaporationand further dried under high vacuum. Product was purified using silica column chromatographywith 20% ethyl acetate in hexanes as the eluent. 10 (major regioisomer) (3.183 g, 13.6 mmol, 79%).Elem. anal. for C8H9N3OCl2: C, 41.05 (found 42.27); H, 3.88 (found 4.06). 1H-NMR (300 MHz, CDCl3) δ 6.40 (s, 1H), 3.82-3.70 (m, 4H), 3.65 (m, 4H). HRMS [M]+ calcd. for C8H9N3OCl2, 234.0201; found234.0196. 11 (minor regioisomer) (0.806 g, 3.444 mmol, 20%). 1H-NMR (300 MHz, chloroform-d) δ 6.56 (s, 1H), 3.85-3.60 (m, 8H). 13C-NMR (101 MHz, chloroform-d) δ 161.75, 160.55, 108.31, 66.59, 44.39.HRMS [M]+ calcd. for C8H9N3OCl2, 234.0201; found 234.0196.
References:
Wright, Emily W.;Nelson, Ronald A.;Karpova, Yelena;Kulik, George;Welker, Mark E. [Molecules,2018,vol. 23,# 7,p. 1 - 13]

110-91-8
710 suppliers
$9.00/1g

3764-01-0
398 suppliers
$6.00/5g

10397-13-4
163 suppliers
$11.00/250mg

5414-19-7
204 suppliers
$6.00/5g
56-05-3
447 suppliers
$10.00/1g

10397-13-4
163 suppliers
$11.00/250mg
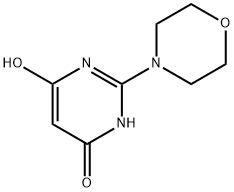
24193-00-8
50 suppliers
$121.00/1g

10397-13-4
163 suppliers
$11.00/250mg
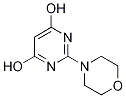
50596-83-3
2 suppliers
inquiry

10397-13-4
163 suppliers
$11.00/250mg