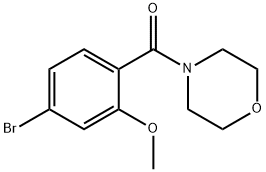
4-[(4-BroMo-2-Methoxyphenyl)carbonyl]Morpholine synthesis
- Product Name:4-[(4-BroMo-2-Methoxyphenyl)carbonyl]Morpholine
- CAS Number:1393441-92-3
- Molecular formula:C12H14BrNO3
- Molecular Weight:300.15

110-91-8
711 suppliers
$9.00/1g
72135-36-5
141 suppliers
$6.00/1g
![4-[(4-BroMo-2-Methoxyphenyl)carbonyl]Morpholine](/CAS/20150408/GIF/1393441-92-3.gif)
1393441-92-3
8 suppliers
$115.00/1g
Yield:1393441-92-3 92%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine;N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate in N,N-dimethyl-formamide at 0; for 1 h;Inert atmosphere;
Steps:
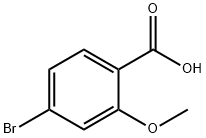
40.1 Step 1: (4-bromo-2-methoxyphenyl)(morpholino)methanone
To DMF (8 mL) were added 4-bromo-2-methoxybenzoic acid (250 mg, 1.08 mmol) and morpholine (0.124 g, 1.41 mmol) under an ice-bath condition. HATU (0.59 g, 1.5 mmol) was added under nitrogen protection, then N,N-diisopropylethylamine (1.1 mL, 6.6 mmol) was added dropwise at 0°C.
After the addition, the mixture was stirred for 1 h under the ice-bath condition.
After the reaction was completed, the mixture was poured into water (50 mL).
The resulting mixture was extracted with EtOAc (25 mL*3).
The combined organic layers were washed with saturated aqueous NaCl solution (20 mL*2), dried over anhydrous Na2SO4, and filtered.
The filtrate was concentrated in vacuo, and the residue was purified by silica-gel column chromatography (DCM:EtOAc=10:1, V/V) to give a white solid (0.30 g, 92 %).
MS (ESI, pos.ion) m/z:301.0 (M+2);
References:
EP3342765,2018,A1 Location in patent:Paragraph 0368