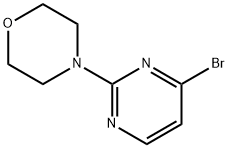
4-(4-BROMOPYRIMIDIN-2-YL)MORPHOLINE synthesis
- Product Name:4-(4-BROMOPYRIMIDIN-2-YL)MORPHOLINE
- CAS Number:663194-10-3
- Molecular formula:C8H10BrN3O
- Molecular Weight:244.09

110-91-8
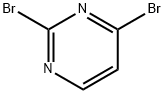
3921-01-5

663194-10-3
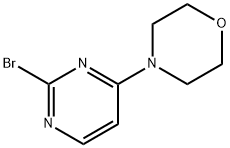
1209459-32-4
A solution of tetrahydrofuran (10 mL) of 2,4-dibromopyrimidine (438.4 mg, 1.84 mmol) and potassium carbonate (1.27 g, 9.21 mmol) was mixed and stirred at room temperature for 5 min. Subsequently, morpholine (174.8 μL, 2.03 mmol) was added dropwise and the reaction mixture continued to be stirred at room temperature for 5 hours. Upon completion of the reaction, the mixture was filtered, the filtrate was collected and the solvent was removed by concentration under reduced pressure. The crude product was purified by silica gel column chromatography using hexane and ethyl acetate as eluents to afford 4-(4-bromopyrimidin-2-yl)morpholine (28a) and 4-(2-bromopyrimidin-4-yl)morpholine (28b) in 19% and 66% yields, respectively. 4-(4-Bromopyrimidin-2-yl)morpholine (28a): white solid in 19% yield.1H NMR (500 MHz, CDCl3) δ ppm 3.74-3.77 (m, 4H), 3.79-3.83 (m, 4H), 6.70 (d, J = 4.88 Hz, 1H), 8.05 (d, J = 4.88 Hz, 1H). LCMS m/z found 246.0, [M + H]+. 4-(2-Bromopyrimidin-4-yl)morpholine (28b): white solid, 66% yield.1H NMR (500 MHz, CDCl3) δ ppm 3.66 (br.s, 4H), 3.76-3.83 (m, 4H), 6.43 (d, J = 6.35 Hz, 1H), 8.02 (d, J = 6.35 Hz, 1H).LCMS m /z found 246.0, [M + H]+.
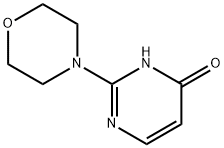
19810-79-8
64 suppliers
$33.00/100mg

663194-10-3
59 suppliers
$70.00/250mg
Yield:663194-10-3 87%
Reaction Conditions:
with phosphorus(V) oxybromide in acetonitrile; for 1 h;Heating / reflux;
Steps:
2c
2c) 4- (4-BROMO-PVRIMIDIN-2-VL)-MORPHOLINE A mixture of 2-morpholin-4-yl-pyrimidin-4-ol (6.08 g, 33.6 MMOL) and phosphorus OXYBROMIDE (12.5 g, 43.7 MMOL) in 330 mL MECN is heated to reflux for 1 hour. The reaction is cooled to room temperature, concentrated to half volume, and poured over ice. The resulting mixture is neutralized with a saturated solution of NaHCOs, and then extracted with methylene chloride. The organic phase is washed with saturated NACI (aqueous), dried over ANHYDROUS MGS04, FILTERED, concentrated and dried to an off-white solid (7.11 g, 87%). M+H'= 245.97. H NMR (CDC13) ; a 8.05 (d, 1H), 6.70 (d, 1H), 3.75 (m, 8H).
References:
WO2005/28444,2005,A1 Location in patent:Page/Page column 50-51

110-91-8
711 suppliers
$9.00/1g

3921-01-5
130 suppliers
$26.00/250mg

663194-10-3
59 suppliers
$70.00/250mg

1209459-32-4
19 suppliers
inquiry