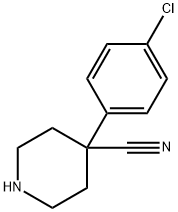
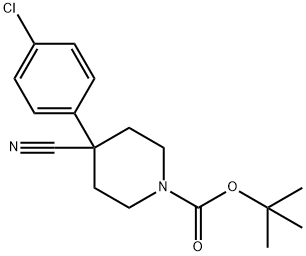
4-(4-CHLOROPHENYL)PIPERIDINE-4-CARBONITRILE synthesis
- Product Name:4-(4-CHLOROPHENYL)PIPERIDINE-4-CARBONITRILE
- CAS Number:91721-16-3
- Molecular formula:C12H13ClN2
- Molecular Weight:220.7
218451-34-4

91721-16-3
The general procedure for the synthesis of 4-(4-chlorophenyl)piperidine-4-carbonitrile from tert-butyl 4-(4-chlorophenyl)-4-cyanopiperidine-1-carboxylate was as follows: tert-butyl 4-(4-chlorophenyl)-4-cyanopiperidine-1-carboxylate (1.8 g, 5.6 mmol) was slowly added hydrochloric acid/methanol solution (20 mL) dropwise to a solution of tert-butyl 4-(4-chlorophenyl)-4-cyanopiperidine-1-carboxylate (1.8 g, 5.6 mmol) in methanol (5 mL) in the conditions of ice bath. After the dropwise addition was completed, the reaction mixture was gradually warmed to room temperature and stirring was continued for 2 hours. Upon completion of the reaction, the mixture was concentrated to remove the solvent and the residue was dissolved in water (30 mL) and washed with ethyl acetate. Subsequently, the pH of the aqueous layer was adjusted to 9 with 1N sodium hydroxide solution and extracted with ethyl acetate (3 x 50 mL). All organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated to give the target product 4-(4-chlorophenyl)piperidine-4-carbonitrile (0.9 g, 73% yield) as a white solid.

218451-34-4
34 suppliers
inquiry

91721-16-3
31 suppliers
inquiry
Yield:91721-16-3 100%
Reaction Conditions:
with trifluoroacetic acid in dichloromethane at 20; for 72 h;
Steps:
30.e e) 4-(4-chlorophenyl)-4-cyanopiperidine
e) 4-(4-chlorophenyl)-4-cyanopiperidine. A solution containing 1-N-Boc-4-(4-chlorophenyl)-4-cyanopiperidine (1.32 g, 4.11 mmol), TFA (11 mL), and DCM (11 mL) was stirred at RT for 72 h, and then concentrated. The residue was treated with water and sat. aq. sodium bicarbonate (until basic), then extracted with DCM (3X). The DCM extracts were dried (Na2SO4), filtered, and concentrated to give the title compound (quant. ) as a colored oil. MS m/z 221 (M+H).
References:
WO2004/22539,2004,A1 Location in patent:Page 34
![4-Piperidinecarbonitrile, 4-(4-chlorophenyl)-1-[(3,4-dimethoxyphenyl)methyl]-](/CAS/20211123/GIF/644981-92-0.gif)
644981-92-0
0 suppliers
inquiry

91721-16-3
31 suppliers
inquiry