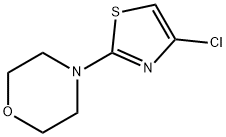
4-(4-CHLOROTHIAZOL-2-YL)MORPHOLINE synthesis
- Product Name:4-(4-CHLOROTHIAZOL-2-YL)MORPHOLINE
- CAS Number:848841-68-9
- Molecular formula:C7H9ClN2OS
- Molecular Weight:204.68

110-91-8
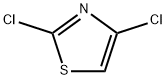
4175-76-2

848841-68-9
A reaction was carried out in acetonitrile (425 mL) with 2,4-dichlorothiazole (34 g, 0.22 mol) and morpholine (21.2 mL, 0.225 mol). Potassium carbonate (60.9 g, 0.44 mol) was first added to the reaction system, followed by slow dropwise addition of morpholine over 30 min. The reaction mixture was refluxed at 40 °C and cooled to 22 °C upon completion. The reaction solution was filtered and the filtrate was evaporated to remove the solvent. The residue was ground with isopropanol (60 mL) at 22 °C for 1 h to promote crystallization. The solid product was collected by filtration and dried under vacuum to constant weight to give the final 4-(4-chlorothiazol-2-yl)morpholine (34.5 g, 76% yield). The product was confirmed by electrospray mass spectrometry (ES/MS), m/z (35Cl) 205 (M + 1)+.

110-91-8
710 suppliers
$9.00/1g

4175-76-2
161 suppliers
$5.00/250mg

848841-68-9
33 suppliers
$65.00/25mg
Yield:848841-68-9 76%
Reaction Conditions:
with potassium carbonate in acetonitrile at 40; for 0.5 h;Heating / reflux;
Steps:
19
Preparation 194-chloro-2-morpholino-thiazoleTo a mixture of 2,4-dichlorothiazole (34 g, 0.22 mol) in acetonitrile (425 mL) add potassium carbonate (60.9 g, 0.44 mol) and then morpholine (21.2 mL, 0.225 mol) dropwise over 30 min. Reflux the mixture at 40 0C and then cool to 22 °C. Filter the mixture and evaporate the filtrate. Triturate the residue with /-propyl alcohol (60 mL) at 22 0C for one hour. Filter the solids and dry under vacuum to a constant weight to afford the title compound (34.5 g, 76%). ES/MS m/z (35Cl) 205 (M+l)+.
References:
WO2008/36579,2008,A1 Location in patent:Page/Page column 37