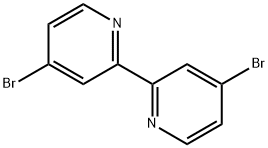
4,4'-DIBROMO-2,2'-BIPYRIDINE synthesis
- Product Name:4,4'-DIBROMO-2,2'-BIPYRIDINE
- CAS Number:18511-71-2
- Molecular formula:C10H6Br2N2
- Molecular Weight:313.98
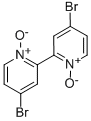
84175-09-7

18511-71-2
The general procedure for the synthesis of 4,4'-dibromo-2,2'-dipyridine using the compound (CAS: 84175-09-7) as starting material was as follows: firstly, 37 g of compound 4-c (0.1 mol) was added to a 2 L round bottom flask under nitrogen protection, followed by the addition of 950 mL of chloroform to dissolve it completely. After cooling the reaction system to -3°C, 297 g of phosphine tribromide (1.1 mol) was slowly added dropwise. After the dropwise addition, the reaction mixture was heated to 60 °C and stirred continuously for 2 hours. After completion of the reaction, the mixture was cooled to room temperature, slowly poured into 1 L of water and the pH was adjusted with sodium hydroxide solution to 11. Subsequently, the organic layer was separated by extraction with dichloromethane. The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure by rotary evaporator to remove the solvent. The resulting solid was washed with ethanol and filtered to give the light yellow solid product 4-d in 26 g yield, giving 77% yield.

84175-09-7
2 suppliers
inquiry

18511-71-2
214 suppliers
$7.00/100mg
Yield:18511-71-2 77%
Reaction Conditions:
with phosphorus tribromide in chloroform at -3 - 60; for 2 h;Inert atmosphere;
Steps:
4.4
A 2 L round-bottom flask was substituted with nitrogen gas, and 37 g of Formula 4-c (0.1 mol) was added thereto, and dissolved by addition of 950 mL of chloroform. At -3° C., 297 g of tribromophosphine (1.1 mol) was slowly dropwise added thereto, followed by stirring at 60° C., for 2 hours. The resultant product was cooled to room temperature, added to 1 L of water, added with caustic soda until pH reached 11, and extracted with methylene chloride. Then, an organic layer was separated. After removal of moisture, and removal of a solvent by vacuum distillation, the precipitated solid was washed with ethanol, and filtered so as to provide a pale yellow solid, 26 g of Formula 4-d (yield 77%).
References:
US2012/247546,2012,A1 Location in patent:Page/Page column 35
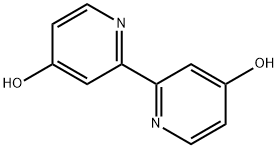
90770-88-0
80 suppliers
$40.00/50mg

18511-71-2
214 suppliers
$7.00/100mg
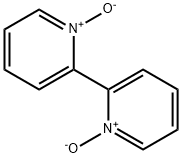
7275-43-6
131 suppliers
$15.00/1g

18511-71-2
214 suppliers
$7.00/100mg
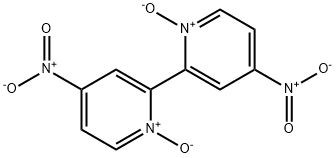
51595-55-2
61 suppliers
$57.75/1g

18511-71-2
214 suppliers
$7.00/100mg
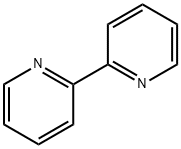
366-18-7
653 suppliers
$5.00/5g

18511-71-2
214 suppliers
$7.00/100mg