
4,4'-didodecyl-2,2'-bithiophene synthesis
- Product Name:4,4'-didodecyl-2,2'-bithiophene
- CAS Number:345633-76-3
- Molecular formula:C32H54S2
- Molecular Weight:502.9
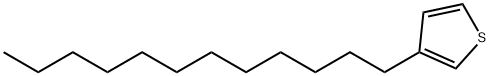
104934-52-3

345633-76-3
General procedure for the synthesis of 4,4'-didodecyl-2,2'-bithiophene from 3-dodecylthiophene: rt-BuLi (7.04 mL, 2.5 M hexane solution) was slowly added dropwise under stirring to a solution containing 3-dodecylthiophene (4.000 g, 15.8 mmol) and N,N,N',N'-tetramethylethylenediamine (2.75 mL, 17.6 mmol) in solution. Subsequently, 80.0 mL of anhydrous ether was added at -78 °C. The reaction mixture was gradually warmed to room temperature and refluxed for 1 hour. Upon completion of the reaction, the mixture was cooled to -78 °C and CuCl2 (2.640 g, 19.6 mmol) was added all at once. The reaction mixture was stirred overnight, during which the temperature naturally increased to room temperature. At the end of the reaction, the reaction was quenched with water and the mixture was extracted with chloroform. The organic layers were combined, washed with water, dried with MgSO4, filtered and concentrated to dryness. The crude product was purified by silica gel column chromatography using hexane as eluent to give a mixture of 4,4'- and 3,3'-bisdodecylthiophene (about 15% of the target product in the mixture as determined by 1H NMR). Finally, purified 4,4'-bidodecyl-2,2'-bithiophene (25) was obtained as a white solid (2.200 g, 55% yield) by recrystallization from a solvent mixture of acetone:ethanol (1:1).1H NMR (CDCl3) data: δ 0.91 (t, 6H), 1.33 (m, 36H), 1.66 (q, 4H), 2.60 (t , 4H), 6.80 (s, 2H), 7.01 (s, 2H) ppm.

51285-60-0
51 suppliers
$49.00/250mg

15890-72-9
47 suppliers
$100.00/100ml

345633-76-3
42 suppliers
inquiry
Yield:345633-76-3 70%
Reaction Conditions:
with 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) in diethyl ether at 20; for 2 h;
Steps:
1.a Example 1
a) A solution of 40 g of 1 in 200 ml of dry tetrahydrofuran (THF) is added rapidly to a solution of lithium diisopropylamide (LDA, prepared from 100 ml of 2.7 M solution of butyllithium in hexane and 28.8 g diisopropylamine in 200 ml of dry THF) at -70° C. under nitrogen atmosphere. After the colour of the mixture has become orange-brown, the mixture is allowed to warm to -20° C. and then 100 ml of water are added. The organic phase is separated, washed with brine, dried and evaporated. The residue is recrystallized from methanol to obtain 36.5 g of 4,4'-dibromo-2,2'-dithiophene as an off-white powder (yield: 91.2%). A solution of n-dodecyl magnesium bromide in ether (prepared from 9 g of magnesium turnings and 87.0 g n-dodecylbromide in 200 ml of diethylether) is slowly added to a solution of 40 g of 4,4'-dibromo-2,2'-dithiophene. 1 mol % NiCl 2(dppp) (dppp=Ph 2PCH 2Ch 2CH 2PPh 2) in 200 ml of diethylether is added in such a way, that the internal temperature does not exceed 20° C. Then the mixture is stirred at room temperature for 2 hours and 200 ml of water are added thereto. The organic phase is separated, washed with diluted hydrochloric acid and brine, dried and evaporated. The residue is suspended in methanol and 55.8 g of 4,4'-n-didodecyl-2,2-dithiophene is obtained as a beige powder by filtation (yield: 70%). 12.8 g of bromine are added dropwise to a solution of 10.1 g 4,4'-n-didodecyl-2,2-dithiophene in 100 ml chloroform and 40 ml acetic acid at 0° C. under nitrogen atmosphere. The mixture is heated at 60° C. for 16 hours. After cooling to room temperature the mixture is treated with 50 ml of a saturated solution of sodium sulfite. The organic phase is separated, washed with a saturated aqueous solution of sodium hydrogen carbonate and brine, dried and evaporated. The residue is suspended in methanol and 14.5 g of 2 is obtained as a beige powder by filtration (yield: 88.4%).
References:
US8436208,2013,B2 Location in patent:Page/Page column 32; 33

104934-52-3
205 suppliers
$7.00/1g

345633-76-3
42 suppliers
inquiry

15890-72-9
47 suppliers
$100.00/100ml

345633-76-3
42 suppliers
inquiry
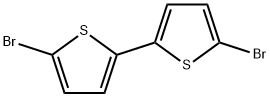
4805-22-5
234 suppliers
$6.00/1g

345633-76-3
42 suppliers
inquiry
