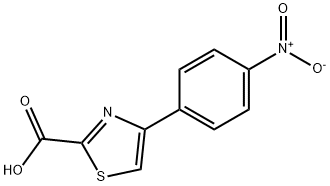
4-(4-Nitrophenyl)thiazole-2-carboxylic Acid synthesis
- Product Name:4-(4-Nitrophenyl)thiazole-2-carboxylic Acid
- CAS Number:4415-05-8
- Molecular formula:C10H6N2O4S
- Molecular Weight:250.23
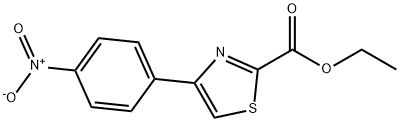
53101-04-5
27 suppliers
inquiry

4415-05-8
14 suppliers
inquiry
Yield:4415-05-8 98%
Reaction Conditions:
Stage #1: ethyl 4-(4-nitrophenyl)-1,3-thiazole-2-carboxylatewith lithium hydroxide monohydrate in tetrahydrofuran at 20; for 4 h;
Stage #2: with hydrogenchloride in water;
Steps:
3.B
To ethyl 4-(4-nitrophenyl)thiazole-2-carboxylate (Intermediate 3, step A, 7.0 g) in THF (70 ml) was added (1 ml) IN lithium hydroxide monohydrate solution and reaction mixture was stirred for 4 hours at RT. Organic solvent was concentrated, water was added, and the reaction mixture was made acidic by dilute HCl solution. This resulted in preciptation of white solid, which was filtered and washed with water. The solid was dried to obtain the title compound. Yield: 6.2 gm (98%). H NMR (DMSO-d6, 300MHz): δ 8.78 (s, 1H), 8.32 (d, 4H).
References:
WO2011/55289,2011,A2 Location in patent:Page/Page column 39
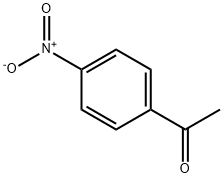
100-19-6
308 suppliers
$5.00/5g

4415-05-8
14 suppliers
inquiry
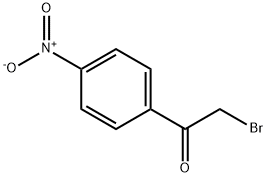
99-81-0
220 suppliers
$9.00/1g

4415-05-8
14 suppliers
inquiry