4-(4-OXO-PIPERIDINE-1-CARBONYL)-BENZAMIDE synthesis
- Product Name:4-(4-OXO-PIPERIDINE-1-CARBONYL)-BENZAMIDE
- CAS Number:204863-53-6
- Molecular formula:C11H11NO3S
- Molecular Weight:237.27
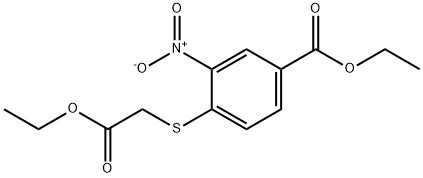
204863-52-5
2 suppliers
inquiry

204863-53-6
26 suppliers
$125.00/1g
Yield:204863-53-6 54%
Reaction Conditions:
Stage #1: ethyl 4-ethoxycarbonylmethylsulfanyl-3-nitrobenzoatewith hydrogen;5%-palladium/activated carbon in ethanol at 50; for 7 h;
Stage #2: with triethylamine in ethanol at 60; for 2 h;
Steps:
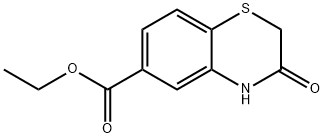
269 Reference Example 269; ethyl 3-OXO-3, 4-dihydro-2H-1, 4-BENZOTHIAZIN-6-CARBOXYLATE
To a solution of ethyl 4- [ (2-ETHOXY-2-OXOETHYL) thio] -3- nitrobenzoate (12.6 g, 40 mmol) in ethanol (120 mL) was added 5% palladium-carbon (1.3 g) and the mixture was stirred under a hydrogen atmosphere (0.5 MPa) at 50°C for 7 hrs. Palladium carbon was collected by filtration, and the filtrate was concentrated. The residue was dissolved in ethanol (100 ML), and triethylamine (7 mL, 50 mmol) was added. The mixture was stirred at 60°C for 2 hrs. The reaction solution was concentrated under reduced pressure and the residue was purified by silica gel column chromatography to give the title compound as pale yellow crystals (5.12 g, yield 54%). 1H NMR (300 MHz, CDC13) 8 ppm: 1.37 (t, J = 7.2 Hz, 3 H), 3.43 (s, 2 H), 4.35 (q, J = 7.2 Hz, 2 H), 7.34 (d, J = 8.0 Hz, 1 H), 7.50 (d, J = 1. 8 Hz, 1 H), 7.64 (J = 8.0, 1. 8 Hz, 1 H), 8.22 (br, 1 H).
References:
WO2004/46107,2004,A1 Location in patent:Page 212-213
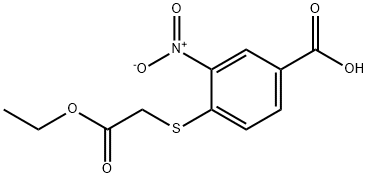
204863-51-4
5 suppliers
inquiry

204863-53-6
26 suppliers
$125.00/1g
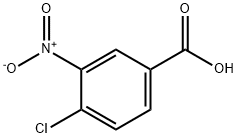
96-99-1
520 suppliers
$5.00/5g

204863-53-6
26 suppliers
$125.00/1g
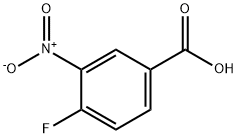
453-71-4
337 suppliers
$5.00/1g

204863-53-6
26 suppliers
$125.00/1g
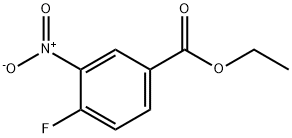
367-80-6
134 suppliers
$22.00/5g

204863-53-6
26 suppliers
$125.00/1g